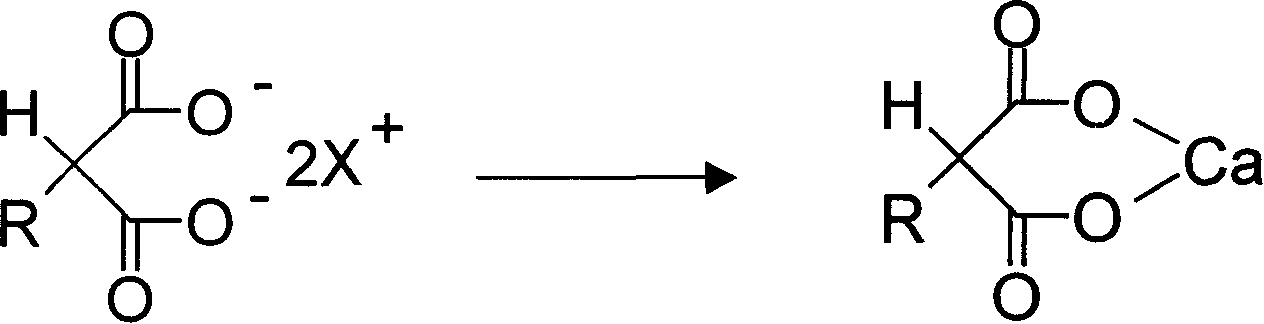
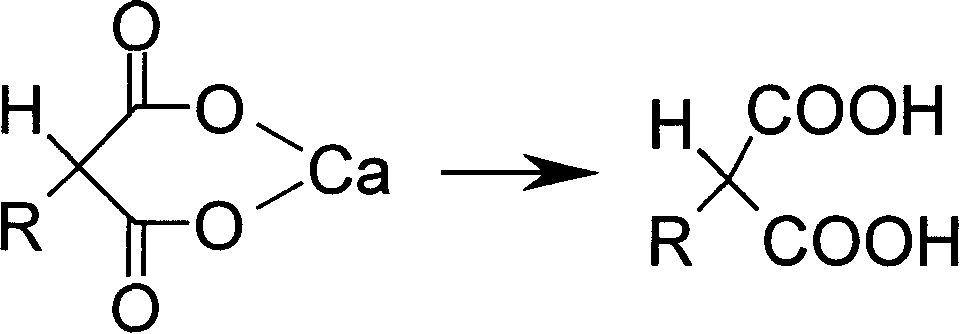
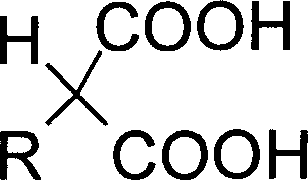
Preparation method of 2 substituted calcium malonate and use of calcium salt
A technology of calcium malonate and its application is applied in the preparation of 2-substituted malonate calcium salt and the application field of calcium salt, which can solve problems such as affecting product purity, shorten the time for recovering or concentrating solvent, prevent decomposition, The effect of simple operation of the separation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 48.4g (0.2mol) of 2-thiophenol diethyl malonate was added to 120g of 20% potassium hydroxide aqueous solution, and hydrolyzed at 90-95° C. for 1-2 hours with TLC (developing solvent: ethyl acetate). : petroleum ether = 1: 5) showed that the spot of the raw material basically disappeared as the reaction end point. Add 65 mL of water and cool to 5°C, add concentrated hydrochloric acid to adjust pH to 6.5, add 0.24 mol of calcium chloride aqueous solution to precipitate a large amount of solid. After filtration, the filter cake was washed with water and methanol, and dried to obtain 39.9 g of white powdery solid of calcium 2-thiophenol malonate with a yield of 89% and a purity of 99.2%.
Embodiment 2
[0034] 48.4g (0.2mol) of 2-thiophenol diethyl malonate was added to 120g of 20% potassium hydroxide aqueous solution, and hydrolyzed at 90-95° C. for 1-2 hours with TLC (developing solvent: ethyl acetate). : petroleum ether = 1: 5) showed that the spot of the raw material basically disappeared as the reaction end point. 65 mL of water was added and cooled to 40° C., and concentrated hydrochloric acid was added to adjust the pH to 7.0, and 0.2 mol of calcium chloride aqueous solution was added to precipitate a large amount of solid. After filtration, the filter cake was washed with water and methanol, and dried to obtain 30.6 g of white powdery solid of calcium 2-thiophenol malonate with a yield of 68.3% and a purity of 99.3%.
Embodiment 3
[0036] 48.4g (0.2mol) of 2-thiophene diethyl malonate was added to 100g of 20% aqueous sodium hydroxide solution, hydrolyzed at 90-95° C. : petroleum ether = 1: 5) showed that the spot of the raw material basically disappeared as the reaction end point. 65 mL of water was added and cooled to 0 °C, and concentrated hydrochloric acid was added to adjust the pH to 6.3, and 0.3 mol of calcium chloride aqueous solution was added to precipitate a large amount of solid. Filter, wash the filter cake with water and methanol, and dry to obtain 37.5 g of white powdery solid of calcium 2-thiophenol malonate with a yield of 83.7% and a purity of 99.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com