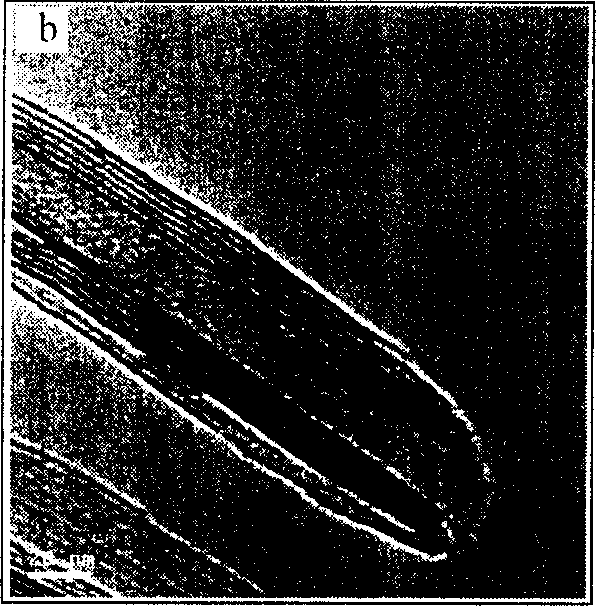
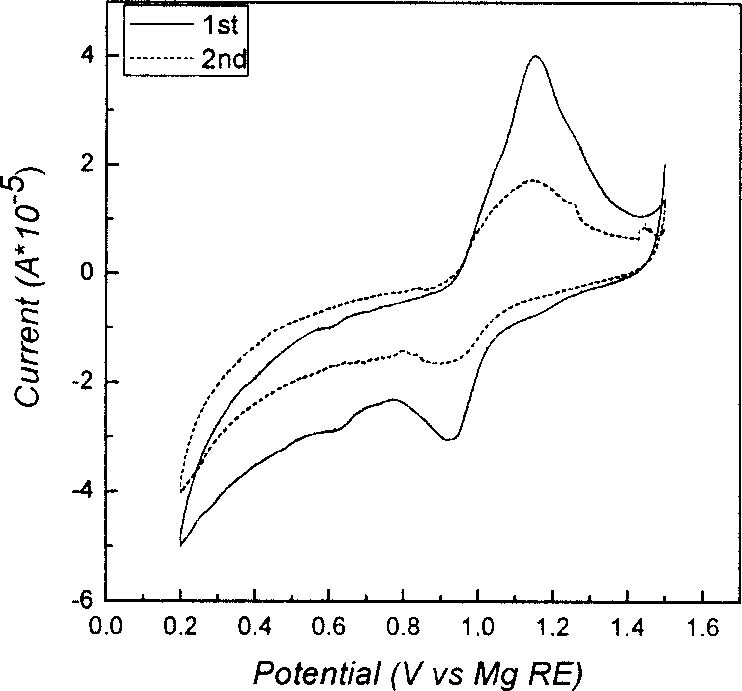
Mg secondary cell
A magnesium secondary battery and electrolyte technology, applied in secondary batteries, secondary battery manufacturing, battery electrodes, etc., to achieve great development and application prospects, broad development prospects and application scope, and improve the effect of discharge specific capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of vanadium oxide nanotubes and their electrochemical performance tests
[0040] Preparation of Vanadium Oxide Nanotubes
[0041] Add 10mmol of V 2 o 5 (A.R. grade) and 10mmol of octadecylamine (A.R. grade, Fluka) were added to 5ml of absolute ethanol and stirred for 2h; then added 15ml of deionized water and continued to stir for 48h; the mixture was transferred to a stainless steel reactor with a polytetrafluoroethylene substrate, React at a constant temperature of 180°C for 7 days; finally, after repeated washing with absolute ethanol and n-hexane, dry in a vacuum oven at 70°C for 6 hours to obtain a black product (VO x -NTs), yield>90%.
[0042] Copper, silver-doped vanadium oxide nanotubes (denoted as: Cu y -doped VO x -NTs, Ag y -doped VO x -NTs) preparation:
[0043] Polycrystalline V 2 o 5 (1g) added to 100ml 10% H 2 o 2 In the middle, the container is cooled with water, and after the solution is stirred for about 2 hours, O 2...
Embodiment 2
[0052] Example 2: Cu 0.1 -doped VO x - Electrochemical properties of NTs
[0053] We assemble the battery condition by embodiment 1 with the Cu prepared by embodiment 1 0.1 Doped vanadium oxide nanotube active materials were assembled into simulated batteries and tested under different discharge current densities. attached Figure 5 It can be seen from the figure that the curve relationship shows that when the battery is discharged at a current density of 10mA / g, the initial discharge capacity is the highest and the cycle efficiency is also good. The undoped vanadium oxide nanotubes have the best cycle performance when the current density is 5mA / g, and the initial discharge capacity is only 81mAh / g, and the doped nanotubes Cu 0.1 -doped VO x The capacity of -NTs can reach 120.2mAh / g when the maximum discharge current density is 10mA / g. It shows that after vanadium oxide nanotubes are doped, the cycle performance and high current discharge performance are improved and enh...
Embodiment 3
[0054] Example 3: Ag y -doped VO x - Electrochemical properties of NTs (y=0.1, 0.2, 0.3)
[0055] We assemble the battery condition by embodiment 1 the Ag that embodiment 1 prepares y -doped VO x - NTs (y=0.1, 0.2, 0.3) active materials were assembled into a simulated battery and tested at a discharge current density of 5mA / g. attached Figure 6 is the initial discharge capacity curve of vanadium oxide nanotubes with different Ag-doped metal contents at a discharge current density of 5 mA / g (A1: Ag 0.1 -dopedVO x -NTs, A2: Ag 0.2 -doped VO x -NTs, A3: Ag 0.3 -doped VO x -NTs), it can be seen from the curve that when the Ag doping percentage is 20mol% of the vanadium oxide, the initial discharge specific capacity of the simulated battery is the highest, reaching 108.4mAh / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com