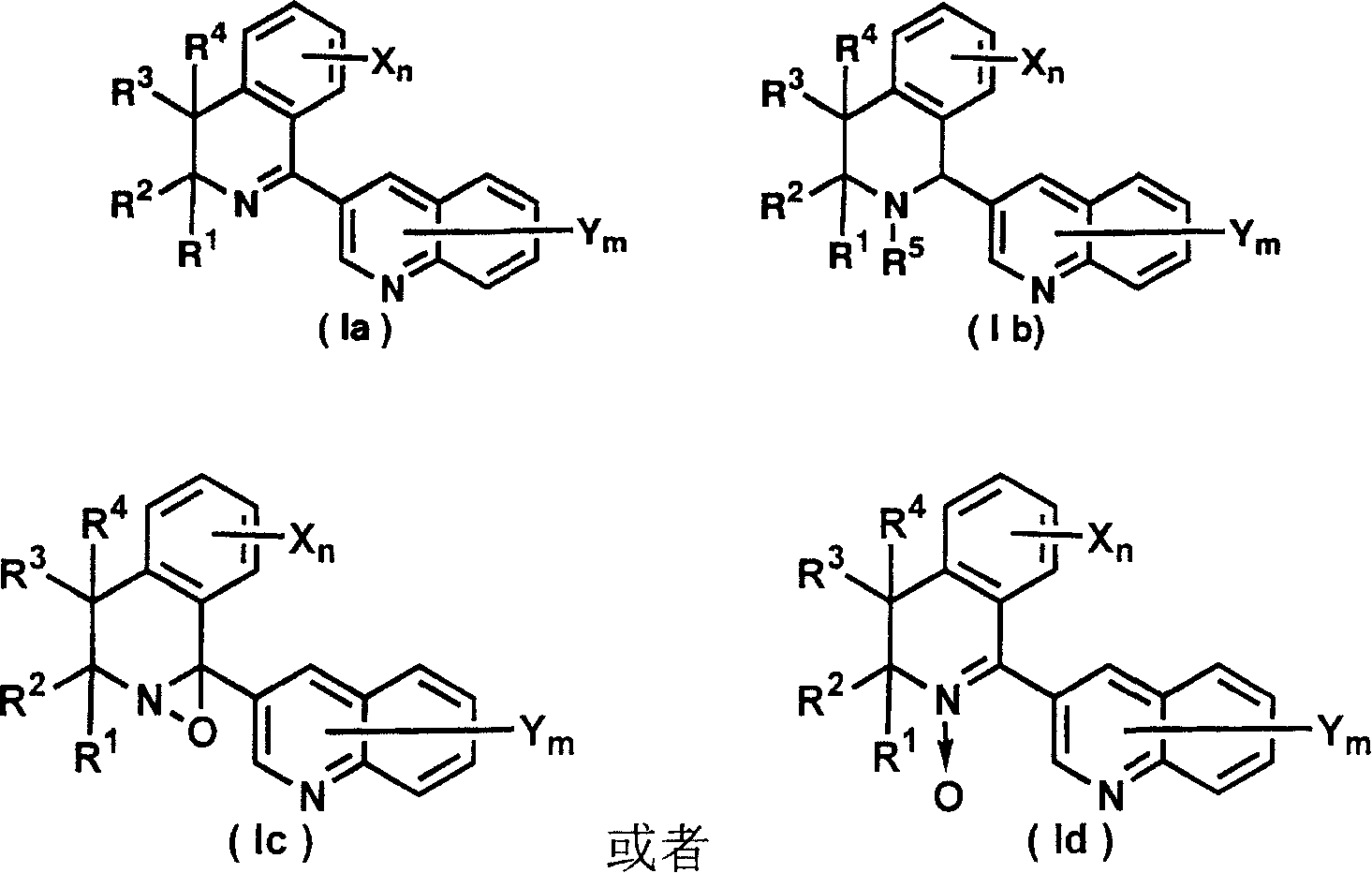
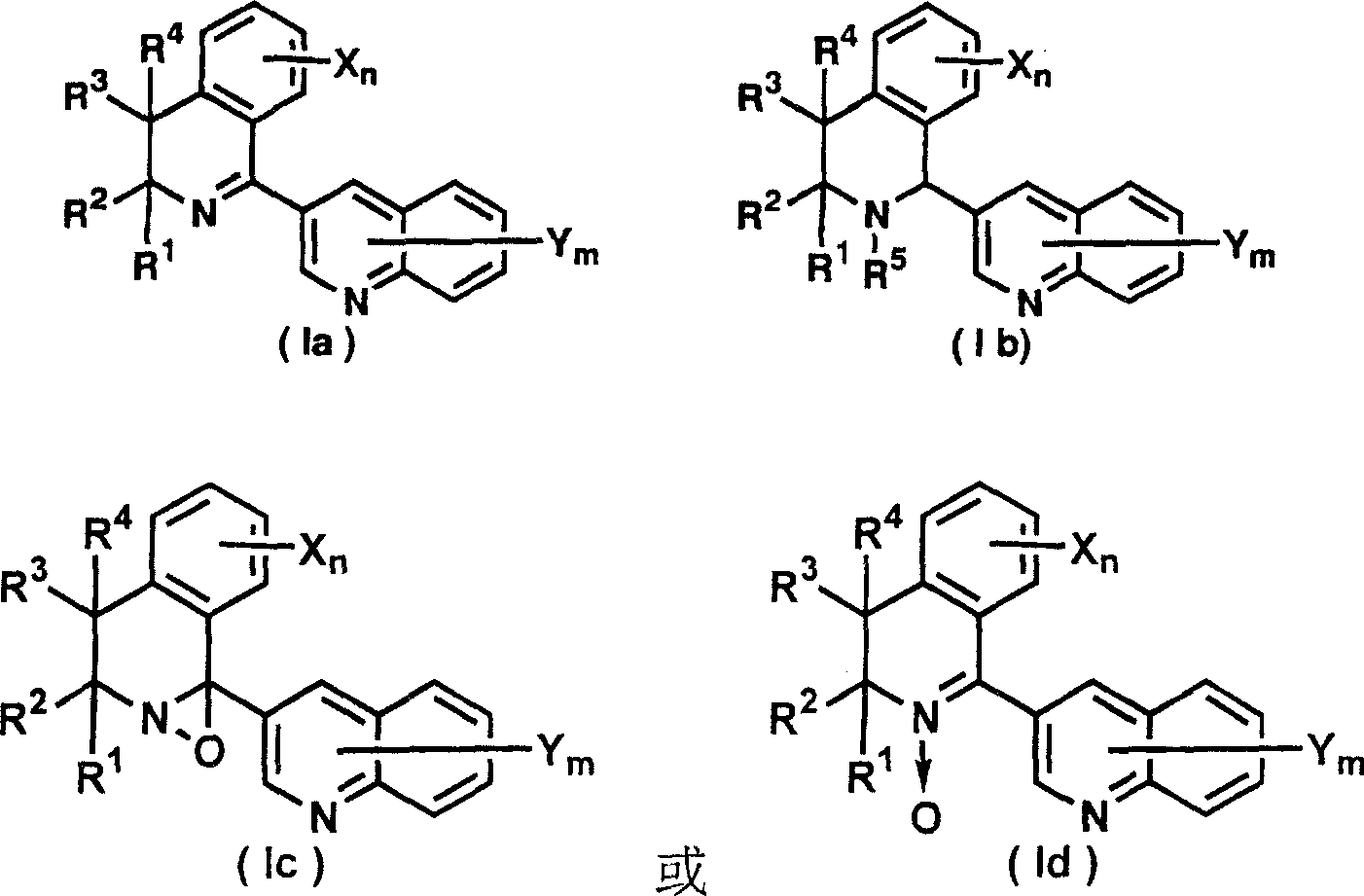
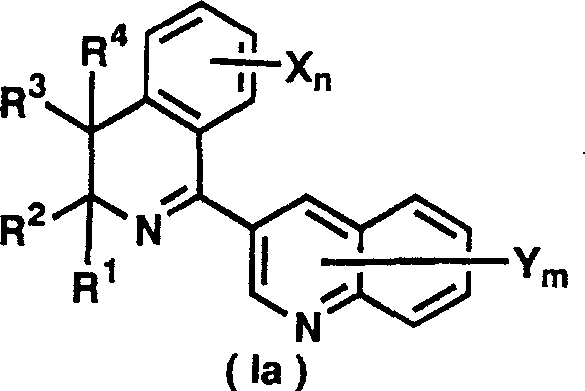
3-(dihydro(tetrahydro)isoquinolin-1-yl)quinolines
A technology of dihydroisoquinoline and compounds, applied in organic chemistry, animal repellent, plant growth regulator, etc. 1-ylquinoline compound, no record, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0294] 6'-Methyl-1'-quinolin-3-yl-4'H-spiro[cyclohexane-1,3'-isoquinoline] (Compound No. 1-772) (Process A)
[0295] Under ice-cooling, to quinoline-3-carbonitrile (154 mg, 1.0 mmol) in benzene (1.0 mL) was added sulfuric acid (0.4 mL) and 1-(3-methylbenzyl) cyclohexanol (204 mg, 1.0 mmol mol), stirred at 80°C for 1 hour, added water, extracted with ethyl acetate, and chromatographically separated the obtained residue to obtain 180 mg of the target compound (yield 73%).
[0296] Physical properties: oily substance.
[0297] 1 H-NMR (270MHz, CDCl 3 )δppm: 1.51-1.85 (10H, m), 2.40 (3H, s), 2.81 (2H, s), 7.02-7.14 (3H, m), 7.57 (1H, t, J=8.4Hz), 7.75 (1H , t, J = 8.4Hz), 7.86 (1H, d, J = 8.4Hz), 8.15 (1H, d, J = 8.4Hz), 8.36 (1H, s), 9.16 (1H, s). MS m / z: 340 (M + ), 325, 311, 297, 284, 244, 142, 128.
Embodiment 2
[0299] 3-(5-Fluoro-3,3-dimethyl-3,4-dihydroisoquinolin-1-yl)quinoline (Compound No. 1-32) (Process A)
[0300] Under ice cooling, add 1-fluoro-(2-methylpropen-1-yl)benzene and 1-fluoro-(2-methylpropen-2-yl)benzene to a mixture of about 4 to 7 (87.3 mg, 0.58 mg mol) and quinoline-3-carbonitrile (89.6mg, 0.58mmol) in dichloroethane (0.58mL), add trifluoromethanesulfonic acid (0.52mL), stir at room temperature for 18 hours, add water, It was extracted with ethyl acetate, and the obtained residue was chromatographed to obtain 82.2 mg of the target compound (yield 47%).
[0301] Melting point: 97~100℃.
[0302] 1 H-NMR (500MHz, CDCl 3 )δppm: 1.36 (6H, s), 2.89 (2H, s), 7.03 (1H, dd, J = 1.4, 6.9Hz) 7.18-7.24 (2H, m), 7.60 (1H, t, J = 8.2Hz) , 7.77 (1H, ddd, J = 1.3, 6.9, 8.2Hz), 7.88 (1H, d, J = 8.2Hz), 8.16 (1H, d, J = 8.2Hz), 8.36 (1H, d, J = 2.1 Hz), 9.09 (1H, d, J=2.1Hz).
[0303] MS m / z: 304 (M + ), 303, 289, 248, 156.
Embodiment 3
[0305] 3-(5-Acetyl-3,3-dimethyl-3,4-dihydroisoquinolin-1-yl)quinoline (Compound No. 1-114) (Step B)
[0306] To a solution of 3-(5-bromo-3,3-dimethyl-3,4-dihydroisoquinolin-1-yl)quinoline (806 mg, 2.2 mmol) in toluene (0.9 mL), tris Butyl(1-ethoxyvinyl)tin (0.85mL, 2.4mmol) and dichlorobis(triphenylphosphine)coordinated palladium (15.8mg, 0.022mmol), after stirring at 100°C for 3 hours, Add dilute hydrochloric acid to temporarily make it acidic, then make it alkaline with ammonia water, filter, concentrate the filtrate, and chromatographically separate the obtained residue to obtain 647 mg of the target compound (yield 89%).
[0307] Physical properties: oily substance.
[0308] 1 H-NMR (500MHz, CDCl 3 )δppm: 1.31 (6H, s), 2.67 (3H, s), 3.13 (2H, s), 7.32 (1H, t, J = 7.6Hz), 7.37 (1H, dd, J = 1.4, 7.6Hz), 7.60 (1H, ddd, J=1.4, 6.9, 8.2Hz), 7.78 (1H, ddd, J=1.4, 6.9, 8.2Hz), 7.82 (1H, ddd, J=1.4, 7.6Hz), 7.87 (1H, d, J=8.2Hz), 8.16(1H, d, J=8.2Hz), 8.35(1H, d, J=2.1Hz), 9....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com