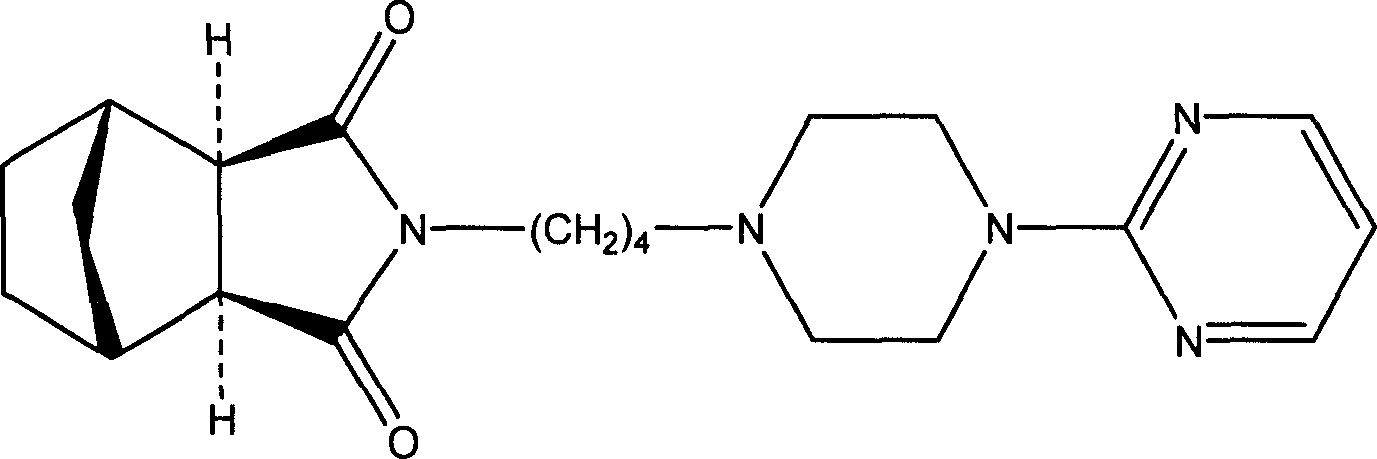
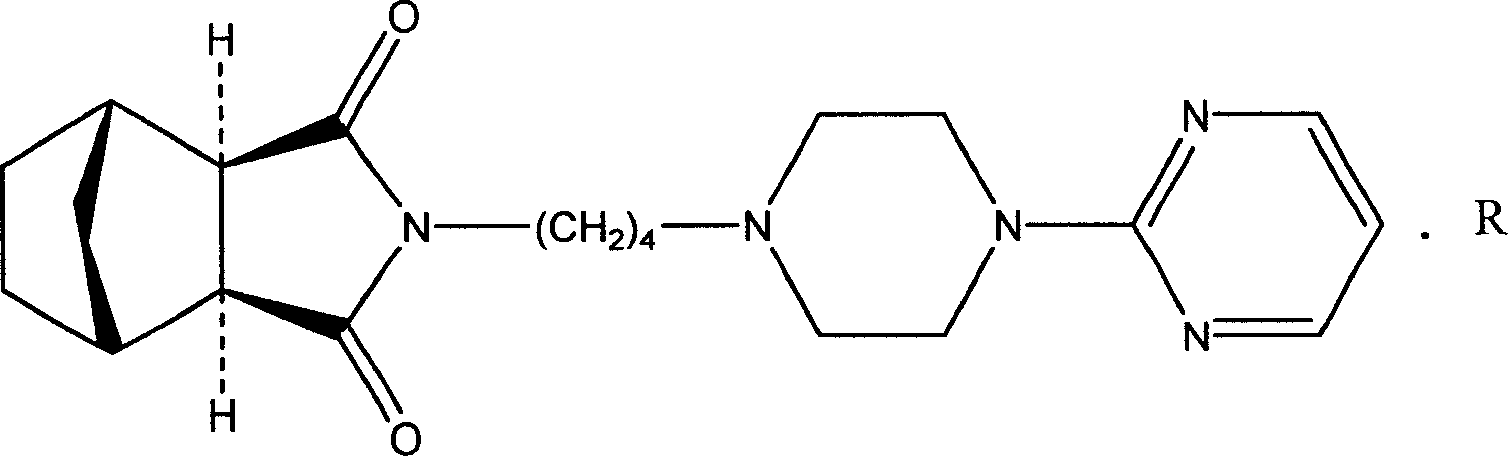
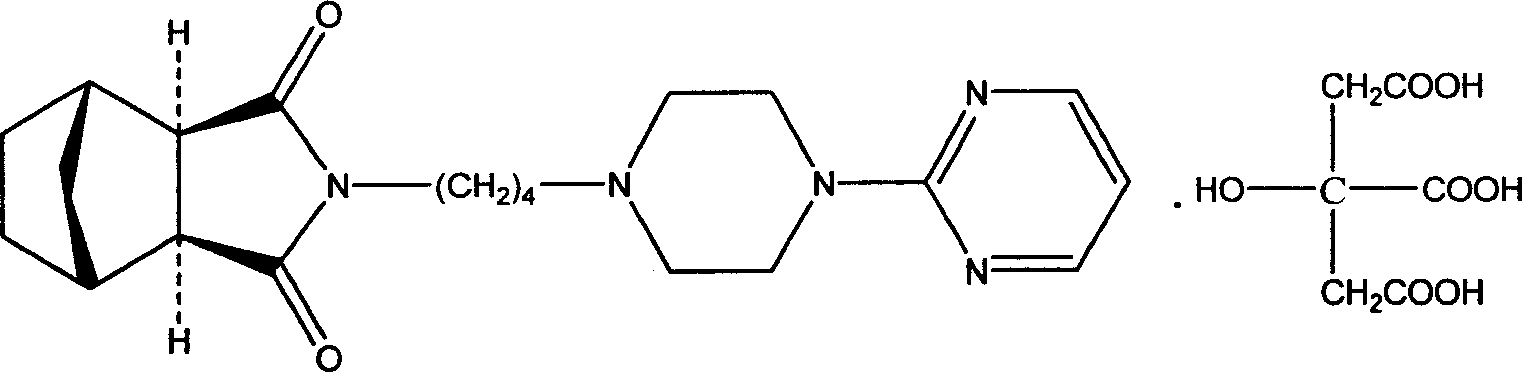
Medicine composition for treating anxiety neurosis, and oral cavity disintegration preparation
An anxiety disorder and composition technology, applied in the field of pharmaceutical composition for treating anxiety disorder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The capsule of embodiment 1 tandospirone (1000 capsules)
[0071] Tandospirone 3g (about 0.008mol)
[0072] Starch 200g
[0073] Microcrystalline Cellulose 5g
[0074] After the tandospirone hydrochloride and the starch are uniformly mixed by an equal increase method, then mixed with the microcrystalline cellulose, granulated, and packed into capsules, the capsules are obtained.
Embodiment 2
[0075] Example 2 Granules of Tandospirone Citrate (1000 Bags of Granules)
[0076] Tandospirone citrate 10g (about 0.17mol)
[0077] Lactose 730g
[0078] Mannitol 230g
[0079] 70% ethanol about 20ml
[0080] Stevioside 10g
[0081] Preparation method: Mix tandospirone citrate, lactose starch and mannitol by equal increase method, then mix with other excipients, and granulate.
Embodiment 3
[0082] Example 3 Granules of Tandospirone Citrate (1000 Bags of Granules)
[0083] Tandospirone citrate 5g (about 0.008mol)
[0084] Starch 2000g
[0085] Powdered sugar 2000g
[0086] Dextrin 2000g
[0087] Soluble starch 4000g
[0088] Stevioside 10g
[0089] Preparation method: Mix tandospirone citrate and dextrin by equal volume increasing method, then mix with other excipients, and granulate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com