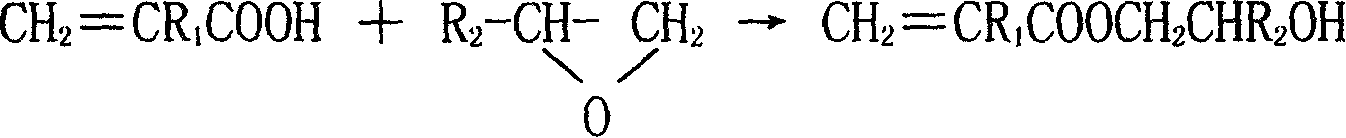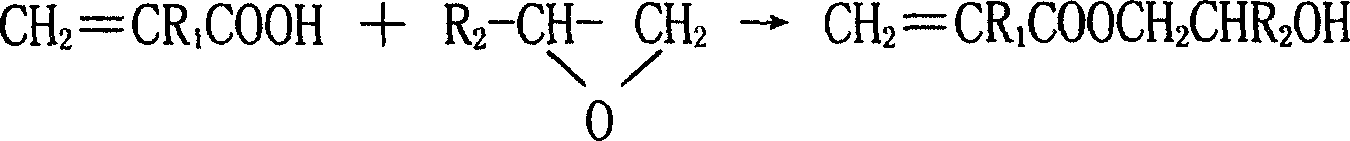Method for synthesizing acrylic acid and hydroxyalkyl methacrylate
A technology of hydroxyalkyl methacrylate and synthesis method, which is applied in the synthesis of acrylic acid and hydroxyethyl methacrylate, and in the field of synthesis of acrylic acid and hydroxyalkyl methacrylate, which can solve the problems of large environmental pollution, increased side reactions, High reaction temperature and other problems, to achieve the effect of low environmental pollution, less by-products and light color
Active Publication Date: 2007-02-28
NANJING FORESTRY UNIV +1
View PDF0 Cites 14 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0006] 3. Ethylene (propylene) glycol reacts with (meth)acryloyl chloride to produce hydroxyethyl (meth)acrylate. Since the cost of acid chloride is much higher than that of the corresponding carboxylic acid, it has great potential in industrial application. limitation
[0007] 4. The reaction between ethylene (propylene) glycol carbonate and (meth)acrylic acid can produce (meth) hydroxyethyl (propyl) acrylate. This method has a longer reaction time and a higher reaction temperature. Long time and high temperature will cause side reactions Increase
[0008] 5. Chlorohydrin method, that is, sodium (potassium) acrylate and ethyl chloride (propanol) react to produce hydroxyethyl (propyl) (meth)acrylate. This method has the filtration problem of by-product NaCl and chlorohydrin Disadvantages of high toxicity
However, this type of catalyst is more polluting to the environment, and some need to be used in combination with special co-catalysts and polymerization inhibitors, and it is difficult to recover and treat the chromium in the raffinate after refining
[0012] 2. Iron-based catalysts have good catalytic performance (activity and selectivity), but are poorer than chromium-based catalysts
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
example 1
[0027] composition
example 2
[0029] composition
example 3
[0031] composition
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a synthesizing method of acroleic acid and methacrylate hydroxyalkyl ester, adopting acroleic acid or methacrylate as raw material; proceeding additional reaction for epoxyethane or epoxypropane with molar rate at 1-1.5: 1 to produce (methyl) acroleic hydroxyethyl (hydroxypropyl) ester; setting the reacting temperature at 60-120 deg.c; using the quantity of catalyst at 0.1-1.0% as reactant.
Description
technical field [0001] The present invention relates to a kind of synthesis method of acrylic acid and hydroxyalkyl methacrylate, in particular to a kind of synthesis method of acrylic acid and hydroxyethyl (or propyl) methacrylate, acrylic acid and hydroxyethyl (or propyl) methacrylate The chemical name of the ester is acrylic acid and methacrylic acid-β-hydroxyethyl (or propyl) ester, and the structural formula is: CH 2 =CR 1 COOCH 2 CHR 2 OH(R 1 , R 2 = H, CH 3 ). It belongs to the field of organic chemical technology. Background technique [0002] Acrylic acid and hydroxyalkyl methacrylate mainly include hydroxyethyl acrylate, hydroxypropyl acrylate, hydroxyethyl methacrylate and hydroxypropyl methacrylate. It is a colorless and transparent liquid with two active Functional group, carbon-carbon double bond and hydroxyl group, is a highly reactive functional monomer, which has an important use in the synthesis of acrylic resin coatings with excellent performance, ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07C69/54C07C67/08
Inventor 朱新宝尹佳子刘准
Owner NANJING FORESTRY UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com