Preparation method of high purity serum gonadotrophin
A production method and high-purity technology, applied in the field of biopharmaceuticals, to achieve the effects of high purification efficiency, less loss, and easy mastery of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
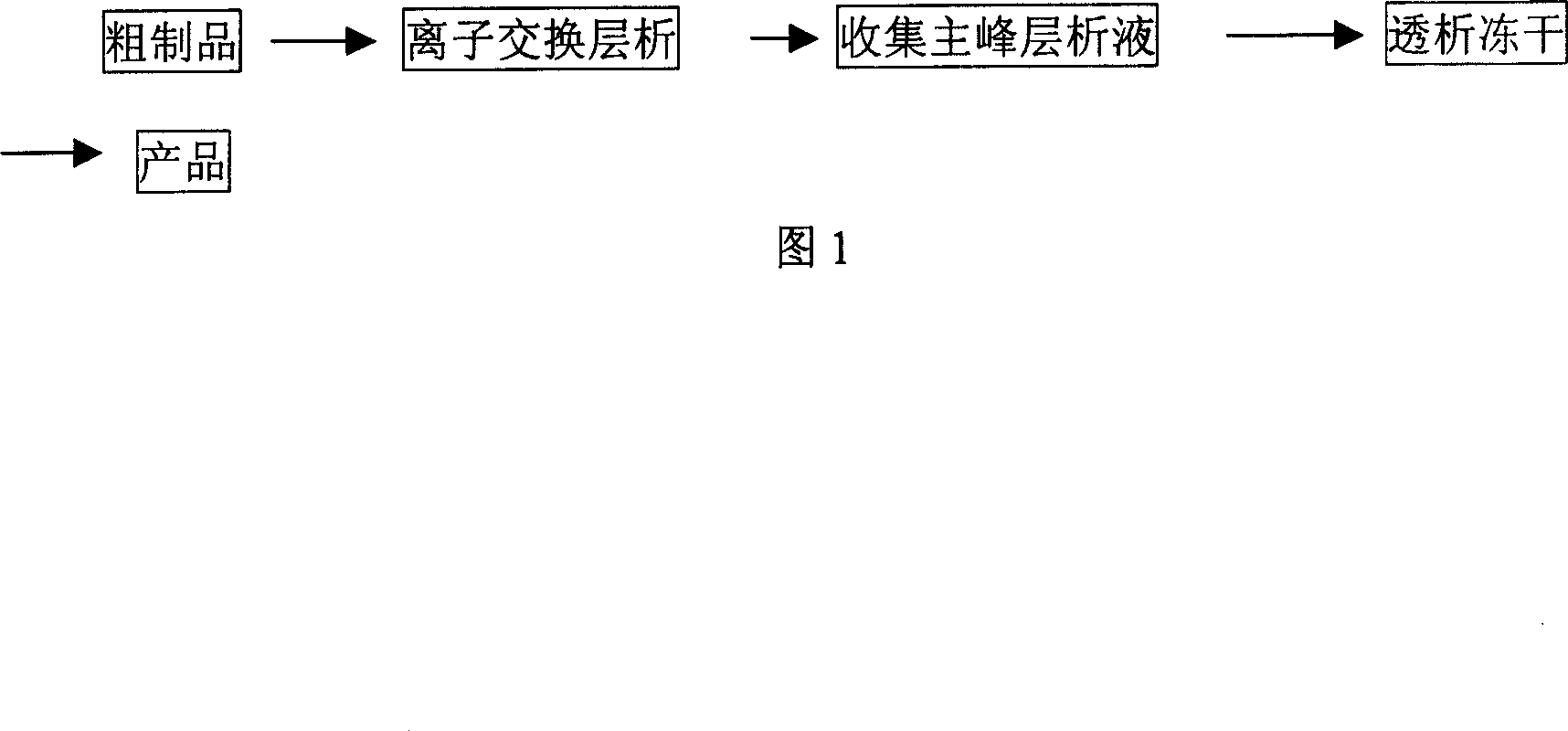
[0075] DEAE-Sephadex A-50 is an ion-exchange chromatography medium, first soaked in water for injection for 24 hours, then equilibrated to pH7.0 with initial buffer solution (0.01mol / L sodium acetate buffer solution of pH7.0), and loaded after equilibrium Column, the column is φ50mm×500mm, equilibrated with the initial buffer again.
[0076] The sample volume is about 2 million u of the crude product (potency is about 800 units / mg), and the crude product is dissolved in 100ml of 0.01mol / L sodium acetate buffer solution with a pH of 7.0, and the sample is slowly added dropwise after it is completely dissolved.
[0077] The amount of each gradient eluent is 3 times the volume of the column. Under the conditions of a flow rate of 10ml / min and a detection wavelength of 280nm, sequentially use the first gradient eluent (0.01mol / L sodium acetate buffer at pH 7.0) , the second gradient buffer (0.05mol / L sodium acetate buffer of pH7.0) and the third gradient buffer (0.2mol / L sodium ac...
preparation example 2
[0080] DEAE-Sephadex A-50 is an ion-exchange chromatography medium, which is first soaked in water for injection for 24 hours, then balanced to pH6.5 with an initial buffer solution (0.005mol / L sodium acetate buffer solution of pH6.5), and loaded Column, the column is φ50mm×500mm, equilibrated with the initial buffer solution again.
[0081] The sample volume is about 1.5 million u crude product (potency is about 650 units / mg), the crude product is dissolved in the initial buffer solution, and the sample is slowly added dropwise after complete dissolution.
[0082] The amount of each gradient eluent is 4 times the volume of the column. Under the conditions of a flow rate of 10ml / min and a detection wavelength of 280nm, the first gradient eluent (initial buffer solution) and the second gradient buffer (pH6. 5 0.07mol / L sodium acetate buffer) and the third gradient buffer (pH6.5 0.3mol / L sodium acetate buffer) elution process, detected by the nucleic acid protein detector, colle...
preparation example 3
[0085] DEAE-Sephadex A-50 is an ion-exchange chromatography medium, which is first soaked in water for injection for 24 hours, then balanced to pH8.5 with an initial buffer solution (0.015mol / L sodium acetate buffer solution of pH8.5), and loaded Column, the column is φ50mm×300mm, equilibrated with the initial buffer solution again.
[0086] The sample volume is about 2 million u crude product (potency is about 860 units / mg), the crude product is dissolved with the initial buffer solution, and the sample is slowly added dropwise after complete dissolution.
[0087] The amount of each gradient eluent is twice the volume of the column. Under the conditions of a flow rate of 10ml / min and a detection wavelength of 280nm, the first gradient eluent (initial buffer solution) and the second gradient buffer (pH8. 5 0.025mol / L sodium acetate buffer) and the third gradient buffer (0.15mol / L sodium acetate buffer of pH 8.5) during the elution process, after detection by a nucleic acid pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com