Reconstruction of HIV-1 Chinese epidemic strain gag gene and recombination DNA vaccine thereof
A DNA vaccine and gene technology, applied in the direction of recombinant DNA technology, plant gene improvement, gene therapy, etc., can solve the problems of long expression time and unsuitable application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Preparation of the modified gag gene (mod.gag)
[0045] In this example, through codon optimization, a modified gag gene (mod.gag) capable of expressing in eukaryotic cells independent of the regulatory protein Rev was obtained.
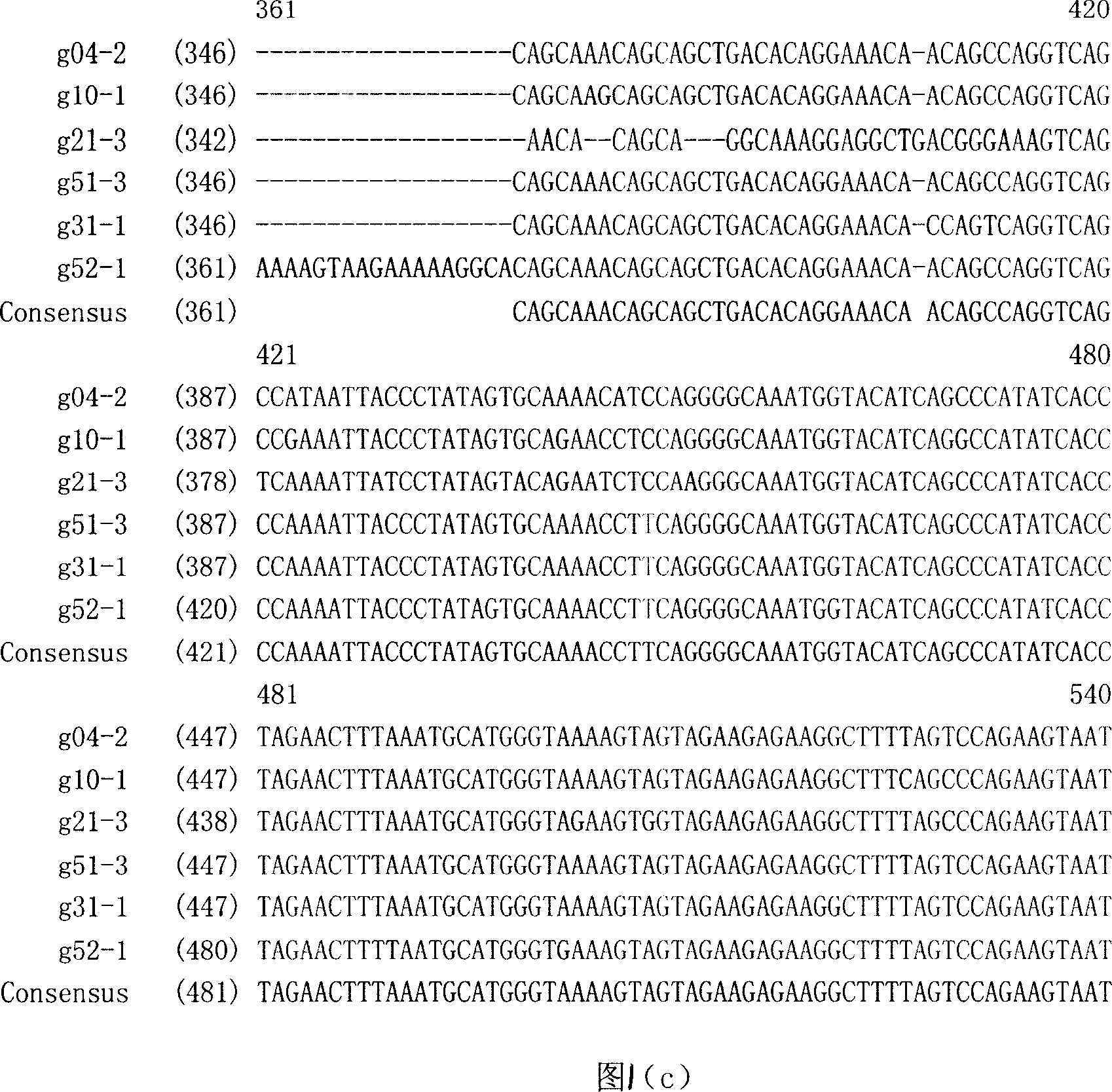
[0046] 1.1 Cloning of HIV-1 gag gene and acquisition of consensus sequence
[0047] Collect 20 samples of venous blood from HIV-1 antibody-positive patients in HIV-1 endemic areas in Henan, 5ml each, anticoagulate with heparin, use Qiagen’s QiaAmp Blood reagent, extract cellular DNA according to the instructions, and store nucleic acid samples at -20°C. The gag gene was amplified by nested-PCR method. Use G-1 / G-2 (G-1: 5'CGA CGC AGG ACT CGG CTT GC 3'; G-2: 5'CCT GGCTTT AAT TTT AC 3') as the outer primers for the first PCR reaction, The conditions are: pre-denaturation at 94°C for 5 min, 30 cycles at 94°C for 45 sec, 55°C for 45 sec, and 72°C for 210 sec; 72°C for 10 min. Take one tenth of the PCR product and us...
Embodiment 2
[0060] Example 2 Construction and Identification of DNA Vaccine pVR-mod.gag Containing mod.gag Gene
[0061] Considering that the final purpose of the constructed DNA vaccine is to be applied to clinical trials, the present invention uses a plasmid vector pVR (gifted by Professor Kong Wei of Jilin University) that can be applied to humans. This vector uses kanamycin resistance instead of general The ampicillin resistance screening marker commonly used in the vector can screen positive clones in Escherichia coli, and does not contain any eukaryotic screening markers, which ensures its safety in the human body.
[0062] 2.1 Construction and identification of recombinant plasmid containing mod.gag
[0063] template
Primer G1
Primer G2
dNTP (2.5mM)
10×PCR buffer
Taq enzyme
h 2 o
2μl
2μl
2μl
4μl
5μl
2μl (5U)
33μl
total capacity
50μl
[0064] The amplification conditions were...
Embodiment 3
[0069] Example 3 The immune protection test of DNA vaccine
[0070] 3.1 Immunization of DNA vaccine pVR-mod.gag
[0071] Thirty female BALB / c mice, 4-6 weeks old, weighing about 18-25 grams, were randomly divided into 2 groups (vaccine group and control group), 15 mice in each group. The mice in the vaccine group were injected with 100 μl / mouse (100 μg) of pVR-mod.gag into the unilateral tibialis anterior muscle, and each mouse in the control group was injected with an equal volume of PBS in the same manner as a control. The levels of specific cellular immune response and antibody response were detected at 4th, 8th and 12th weeks after the initial immunization, respectively.
[0072] 3.2 Detection of HIV-1 gag-specific cellular immune response in immunized mice by intracellular cytokine staining (ICS)
[0073] At 4 weeks, 8 weeks and 12 weeks after the initial immunization, the mice were killed by cervical dislocation, and the spleen lymphocytes of the m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com