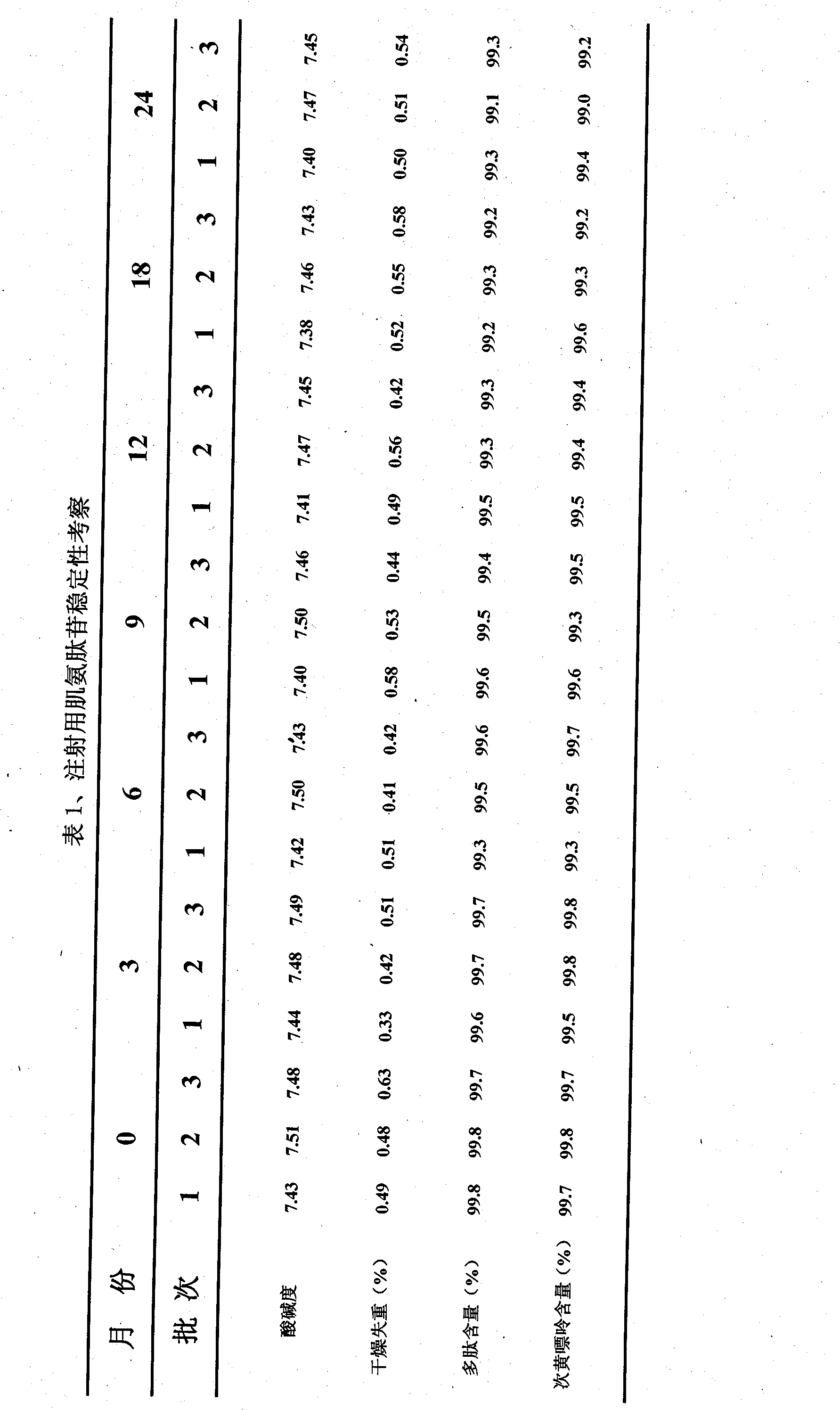
Injection sarcosine peptide aglycone powdery injection and its making method
A technology of carnosine glycoside powder injection and carnosine glycoside polypeptide, which is applied in the field of carnosine glycoside powder injection for injection and its preparation, can solve the problems such as poor extraction effect of hypoxanthine, and achieve product quality avoidance and thorough removal , The effect of increasing the yield
Active Publication Date: 2010-09-08
HARBIN SANLIAN PHARMA CO LTD
View PDF2 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The extraction process of the sarcosaminoglycan glycoside solution described in the patents whose application numbers are 03104642.8 and 200310104970.8 respectively has a poor extraction effect on hypoxanthine
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
Description
Technical field: The invention relates to carnosine glycosides, in particular to a carnosine glycoside powder for injection and a preparation method thereof. Background technique: Carnosine glycosides are extracted from healthy rabbit muscle and heart muscle and contain mixtures of polypeptides, amino acids, nucleosides and nucleotides. Nucleotides and various amino acids (essential amino acids) are important substances involved in human life activities. For diseases of the cardiovascular system, it can improve blood circulation disorders, reduce vascular resistance, increase myocardial oxygen utilization, etc. It can promote the activity of the hematopoietic system, increase the number of white blood cells, increase the elasticity of blood vessels, and prevent vascular sclerosis. Clinically, it is mainly used for brain dysfunction, cerebral apoplexy, cerebral hypofunction caused by insufficient blood supply to the brain, and peripheral nerve diseases. Polypeptides, amino ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): A61K38/17A61K35/34A61K9/14A61K9/19A61P9/10A61P25/02A61P25/00
Inventor 俞嘉林樊宇刘莉丛艳陈芬吴晶晶曲琳娜
Owner HARBIN SANLIAN PHARMA CO LTD
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com