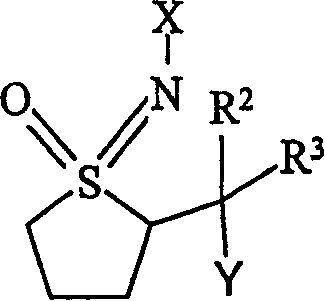
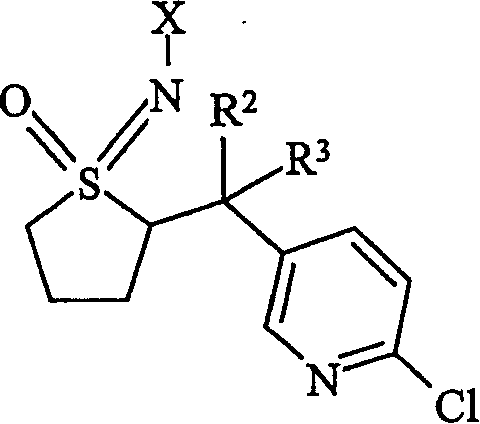
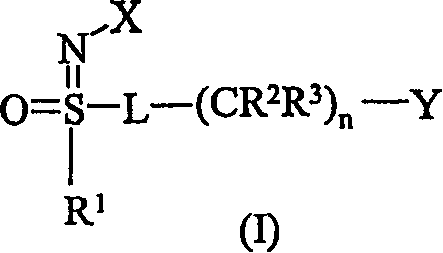Insecticidal N-substituted sulfoximines
A representative, compound technology, applied in the field of N-substituted sulfenimides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I-X
[0065] The preparation of embodiment I-X N-substituted sulfinimide
Embodiment I
[0066] Example I. [3-(6-Chloropyridin-3-yl)-2-methylpropyl](methyl)oxo-λ 4 -sulfanylidenecyanamide (2)
[0067]
[0068] A) Dimethyl 2-[(6-chloropyridin-3-yl)methyl]-2-methylmalonate
[0069]
[0070] To a stirred solution of potassium tert-butoxide (4.49 g, 40 mmol) in tetrahydrofuran (THF, 100 mL) was added dimethyl malonate (6.43 g, 44 mmol) dropwise at room temperature. After 10 minutes, 3-chloromethyl-6-chloropyridine (6.48 g, 40 mmol) was added and the resulting solution was stirred at room temperature overnight. The mixture was poured into water (400 mL), then extracted with ether (2 x 150 mL). The organic fractions were combined, washed with brine (100 mL), and washed with anhydrous MgSO 4 dry. Evaporation of the solvent gave a yellow oil which was triturated with boiling hexane (2 x 100 mL), decanting the hexane from the insoluble oil. The hexane fractions were combined and cooled to give 6.3 g of the desired malonate derivative in 58% yield as a white soli...
Embodiment II
[0095] Example II 2-chloro-5-(2-methyl-3-{methyl(oxo)[oxo-(oxygen)hydrazono]-λ 4 Preparation of -sulfanyl}propyl)pyridine (3)
[0096]
[0097] CH 2 Cl 2 (10 mL) to the stirred solution was added 98% HNO 3 (0.11 g, 1.75 mmol). Separation of the nitrate salt of the sulfinyl imide from solution. To this mixture was added acetic anhydride (4 mL) and a catalytic amount of concentrated H 2 SO 4 (3 drops). The resulting mixture was stirred at 0 °C for several minutes, then heated at reflux for 1 hour. During this time, the reaction mixture became homogeneous. Addition of additional CH to the resulting solution 2 Cl 2 (20 mL), then IN NaOH (75 mL) was added and stirring continued to quench the acetic anhydride. The organic layer was then separated and washed with CH 2 Cl 2 (80 mL) to wash the aqueous layer. The combined organic phases were subjected to MgSO 4 Drying and evaporation of the solvent afforded 0.49 g of product (3) as a 1:1 mixture of diastereomers in 96...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com