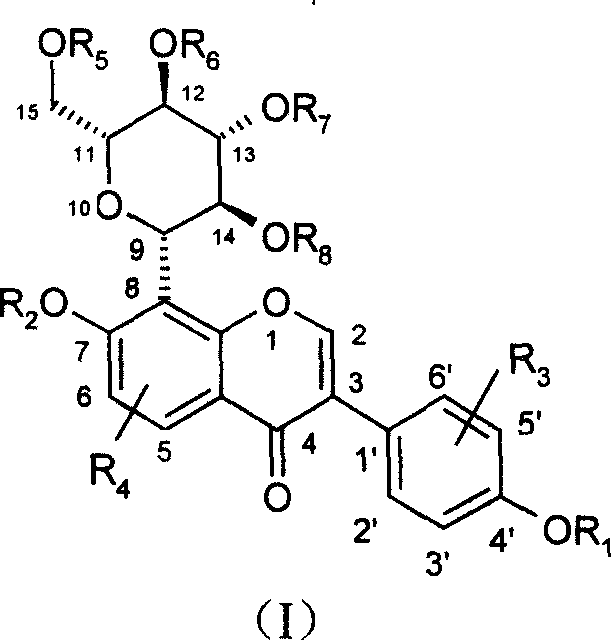
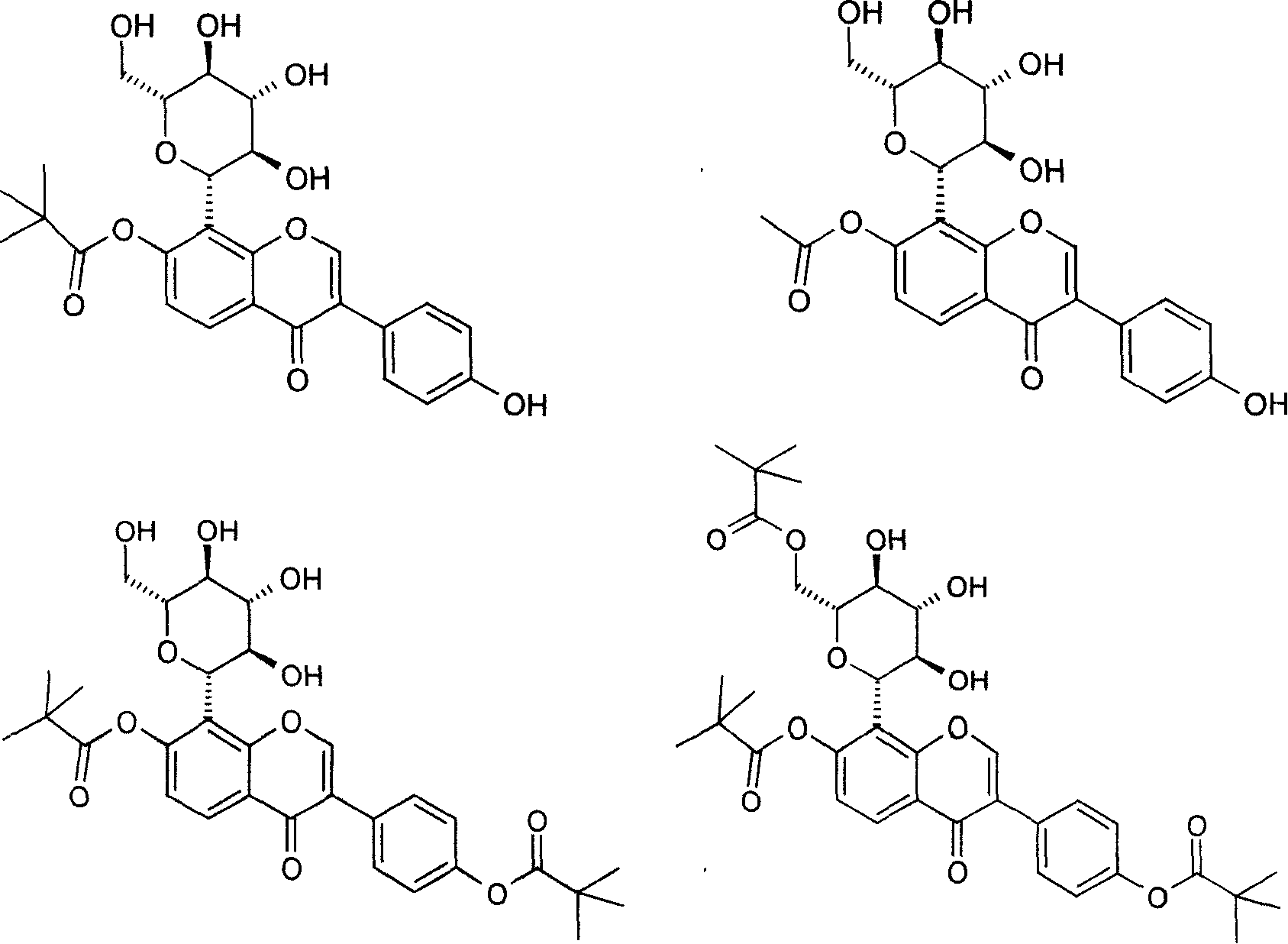
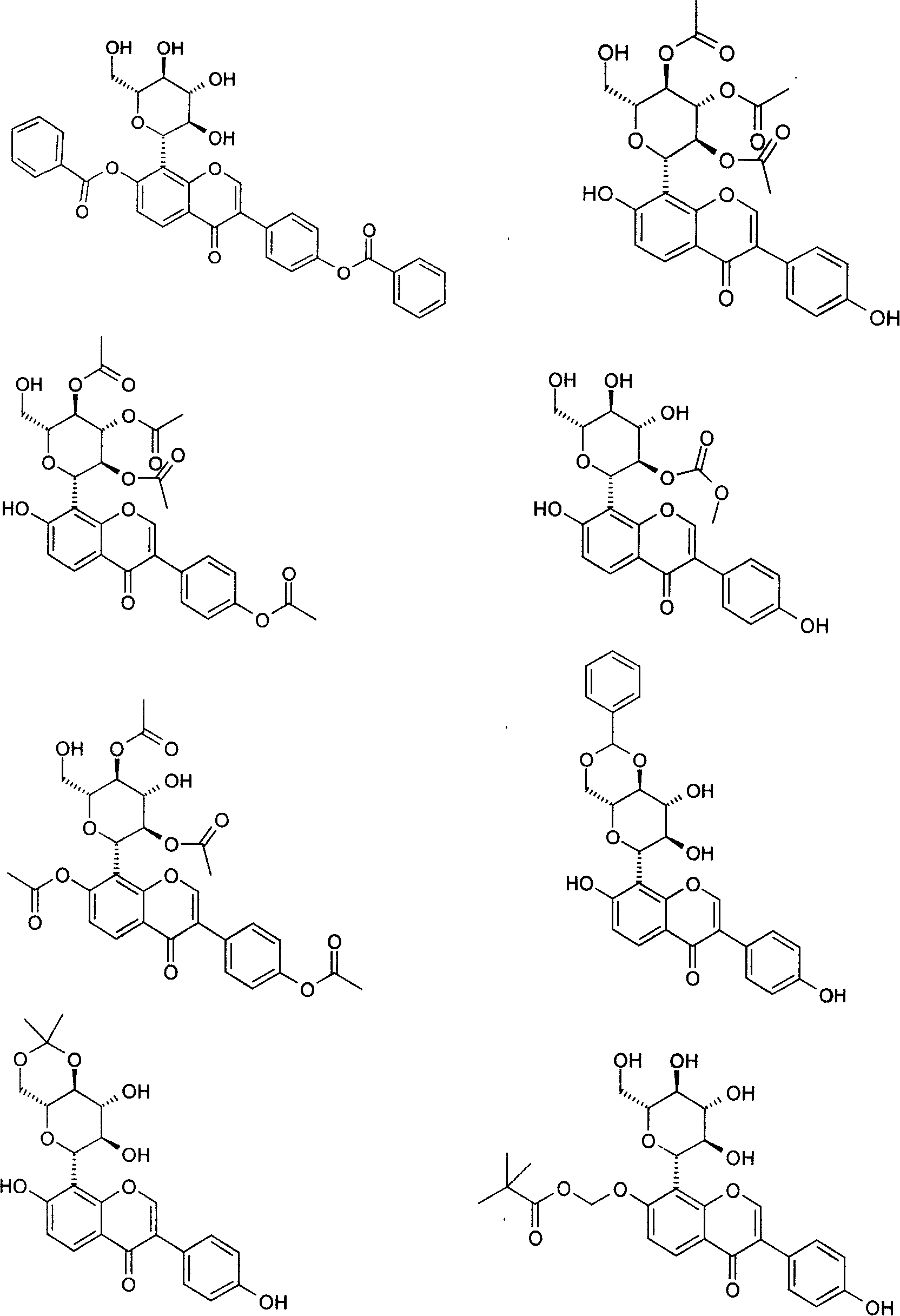
Puerarin derivative and its medicinal use
A technology of puerarin derivatives and halogens, applied in the directions of sugar derivatives, sugar derivatives, drug combinations, etc., can solve problems such as unfavorable crossing of the blood-brain barrier, patient inconvenience, tissue necrosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1.7-O-pivaloyl puerarin
[0051]
[0052]0.416 g (1 mmol) of puerarin was dissolved in 50 ml of acetone, 0.414 g (3 mmol) of anhydrous potassium carbonate was added, and 1 mmol of pivaloyl chloride was added dropwise under stirring at room temperature, and the reaction was tracked by TLC. Stir until the raw material point disappears, spin off most of the solvent, add ethyl acetate to extract, wash with water, dry over anhydrous sodium sulfate, spin off the solvent, column chromatography (ethyl acetate: methanol = 10: 1) to obtain 0.345 g of a white solid product , melting point: 216-218°C, yield 69%.
[0053] 1 H NMR (300MHZ, CD 3 OD), δ: 8.261 (S, 3 / 7H, H2), 8.246 (S, 4 / 7H, H2), 8.17 (d, 1H, H5), 7.35 (d, 2H, H2'6'), 7.165 ( d, 3 / 7H, H6), 7.095 (d, 4 / 7H, H6), 6.80 (d, 2H, H3'5'), 1.37 (S, 9H, -CH 3 ), 5.05(d, 4 / 7H, H9), 4.66(d, 3 / 7H, H9), 4.16(t, 3 / 7H, H14), 3.94(t, 4 / 7H, H14), 3.83(d, 1H, H15), 3.64 (dd, 3 / 7H, H15), 3.54 (dd, 4 / 7H, H15), 3.22-3.52 (m, ...
Embodiment 2
[0054] Example 2.7-O-acetyl puerarin
[0055]
[0056] Dissolve 0.416 g (1 mmol) of puerarin in 50 ml of acetone, add 0.414 g (3 mmol) of anhydrous potassium carbonate, add 1 mmol of acetyl chloride dropwise under stirring at room temperature, follow the reaction by TLC, and stir at room temperature When the raw material point disappeared, most of the solvent was spun off, ethyl acetate was added for extraction, washed with water, dried over anhydrous sodium sulfate, the solvent was spun off, and column chromatography (ethyl acetate:methanol=10:1) gave 0.289 g of a white solid product. Melting point: 194-196°C, yield 63%.
[0057] 1 H NMR (300MHz, CD 3 OD), δ: 8.278 (S, 2 / 5H, H2), 8.246 (S, 3 / 5H, H2), 8.18 (d, 1H, H5), 7.36 (d, 2H, H2'6'), 7.175 ( d, 2 / 5H, H6), 7.15 (d, 3 / 5H, H6), 6.80 (d, 2H, H3'5'), 1.37 (S, 9H, -CH 3 ), 5.05(d, 3 / 5H, H9), 4.67(d, 2 / 5H, H9), 4.15(t, 2 / 5H, H14), 3.93(t, 3 / 5H, H14), 3.83(d, 1H, H15), 3.64 (dd, 2 / 5H, H15), 3.54 (dd, 3 / 5H, H15), 3.3-3.5 ...
Embodiment 3
[0058] Example 3.7-O, 4'-O-dipivaloyl puerarin
[0059]
[0060] Dissolve 0.416 g (1 mmol) of puerarin in 50 ml of acetone, add 0.414 g (3 mmol) of anhydrous potassium carbonate, add dropwise two mmoles of pivaloyl chloride under stirring at room temperature, follow the reaction by TLC, and Stir until the raw material point disappears, spin off most of the solvent, add ethyl acetate to extract, wash with water, dry over anhydrous sodium sulfate, spin off the solvent, column chromatography (ethyl acetate: methanol = 10: 1) to obtain 0.42 g of a white solid product . Melting point: 163-165°C, yield 71%.
[0061] 1 H NMR (300MHz, CDCl 3 ), δ: 8.01 (S, 2 / 3H, H2), 7.99 (S, 1 / 3H, H2), 8.31 (d, 1H, H5), 7.41 (d, 2 / 3H, H2'6'), 7.39 (d, 4 / 3H, H2'6'), 7.11(d, 1 / 3H, H6), 7.02(d, 2 / 3H, H6), 6.85(d, 2H, H3'5'), 5.28(d , 2 / 3H, H9), 4.83(d, 1 / 3H, H9), 5.62(dd, 1 / 3H, H14), 5.39(dd, 2 / 3H, H14), 3.40-4.20(m, 5H, H11 , H12, H13, H15).0.80-1.50(m, 18H, CH 3 ).
[0062] MS (FAB): M+1 = ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com