Preparation method of vitamin D derivative
A technology of derivatives and vitamins, applied in the field of preparing Calcipotriol and Tacalcitol, can solve the problems of poor yield, complicated and cumbersome known processes and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
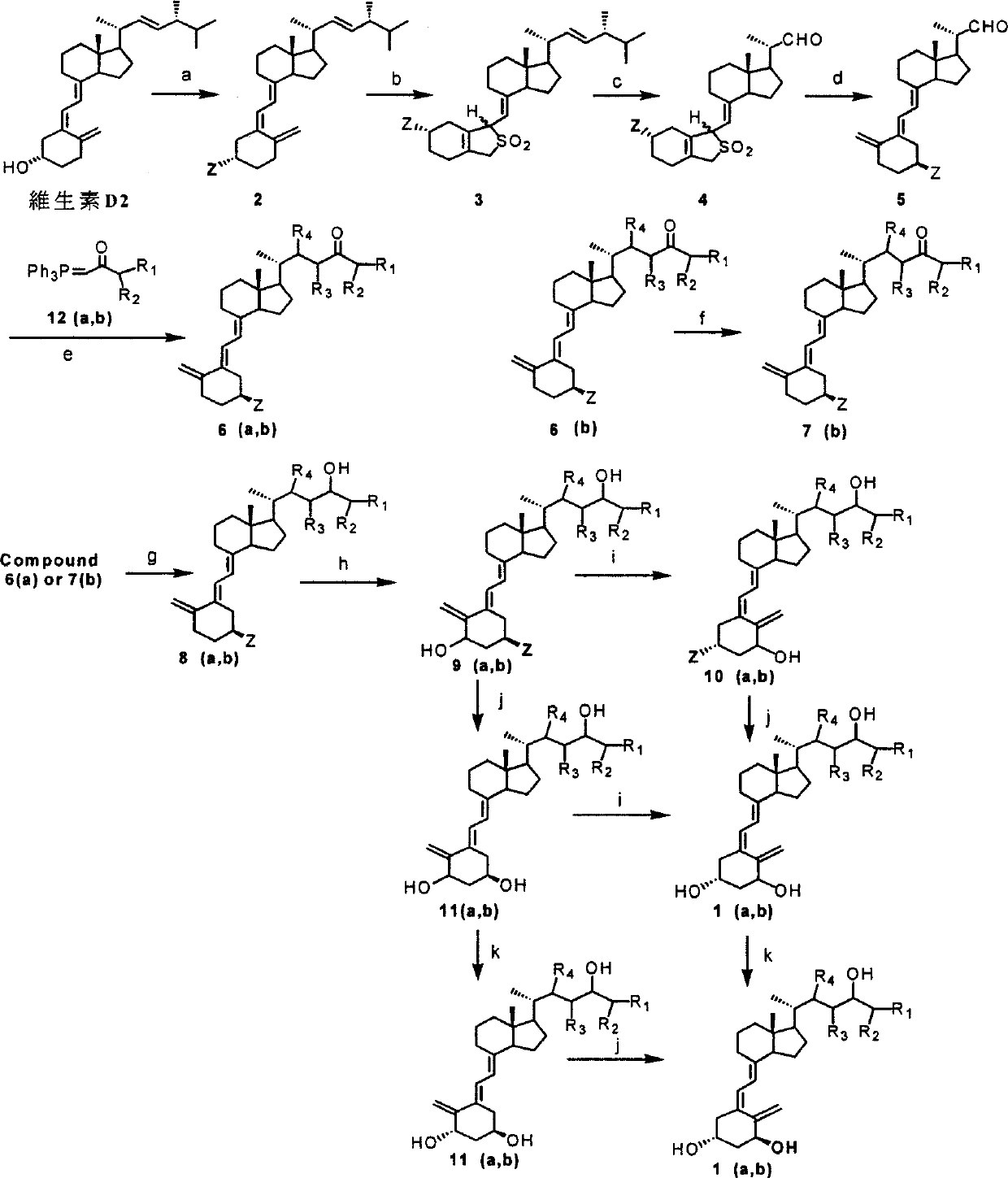
[0090] Embodiment 1: the preparation of compound 2 (Z=t-BuMe 2 SiO)
[0091]First, 2 kg (5.04 mol) of vitamin D2 was provided as a starting material, and then the vitamin D2, 1.16 kg (7.70 mol) of tert-butyldimethylsilyl chloride, and 1.03 kg (15.15 mol) of imidazole were dissolved in 20 liters of dichloromethane solution in, and stirred at room temperature for two hours, after the reaction was completed (detected by thin film chromatography (TLC) sheet, with 10% ethyl acetate in n-hexane solution as developing solution), use 6 liters of water, 6 liters of Aqueous sodium chloride solution, washing with 6 liters of water, and layering steps. The finally obtained organic layer was concentrated under reduced pressure to evaporate the solvent, and about 2.5 kg of the crude product of Compound 2 was obtained. The crude product can be used for the next reaction without further purification.
[0092] This example compound 2 (Z=t-BuMe 2 SiO) 1 H NMR (200MHz, CDCl 3 ) is δ0.07(s,...
Embodiment 2
[0093] Embodiment 2: the preparation of compound 3 (Z=t-BuMe 2 SiO)
[0094] After first adding 2.50 kilograms (4.89mol) of compound 2 prepared in Example 1 into 20 liters of dichloromethane and stirring to dissolve, then add 10 liters of saturated sulfur dioxide (SO 2 ) aqueous solution, and stirred and reacted at room temperature for two hours, after the completion of the reaction (detected by thin film chromatography (TLC) sheet, using 10% ethyl acetate in n-hexane solution as developing solution), SO 2 After distillation, the residue was dissolved in ethyl acetate, washed with water, separated and concentrated to obtain about 2.72 kg of crude compound 3. The crude product can be used for the next reaction without further purification.
Embodiment 3
[0095] Embodiment 3: the preparation of compound 4 (Z=t-BuMe 2 SiO)
[0096] Get 2.72 kilograms (4.73mol) compound 3 crude products prepared in Example 2, be dissolved in the mixed solution of 25 liters of dichloromethane and 2.5 liters of methyl alcohol, and pass into ozone after being cooled to-60 ℃ (O 3 ), detected by thin film chromatography (TLC), using 30% ethyl acetate in n-hexane as a developing solution, when the starting material was almost consumed, the reaction was terminated.
[0097] Filled with nitrogen (N 2 ) to the reaction solution and added 1.6 kg (25.81 mol) of dimethyl sulfide (dimethyl sulfide), then the solution was slowly heated to room temperature to terminate the ozonolysis reaction. Then, add dichloromethane solvent to dilute the solution after the reaction, wash with water, separate layers and concentrate to obtain about 2.20 kg of the crude product of compound 4. The crude product can be used for the next reaction without further purification. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com