Synthesizing porcess for artificial antigen of cyanobromide chrysanthemum ester and assaying process thereof
A technology of artificial antigen and synthesis method, which is applied in the direction of biological testing, material inspection products, etc., can solve the problems that there is no rapid detection method yet, and achieve the effects of convenient and fast processing methods, reducing pollution, and reducing agricultural costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Total synthesis of deltamethrin artificial hapten
[0029] Carry out the total synthesis of deltamethrin artificial hapten according to the following chemical reaction formula:
[0030] 1. Total synthesis of deltamethrin artificial hapten
[0031]
[0032] In the formula:
[0033] 1: Trihydroxybenzaldehyde
[0034] 2: p-Nitrochlorobenzene
[0035] 3: 3-(4-nitrophenoxy)benzaldehyde
[0036]4: [3-(4-nitrophenoxy)phenyl]cyanomethanol
[0037] 5: (1R,3R)-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropylcarboxylate [3-(4-nitrophenoxy)phenyl]cyanomethyl ester
[0038] 6: (1R,3R)-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropylcarboxylate [3-(4-aminophenoxy)phenyl]cyanomethyl ester . 6 is an artificial hapten, which is linked to the protein to obtain the antigen.
[0039] The specific steps are:
[0040] 1), the synthesis of (1R, 3R)-3-(2,2-dibromoethenyl)-2,2-dimethylcyclopropylformyl chloride: add 30g (1R, 3R)- 30ml of chloroform solution of 3-(2,2-dibromovi...
Embodiment 2
[0045] Example 2 Synthesis of deltamethrin artificial antigen
[0046] Carry out according to the following chemical reaction formula:
[0047]
[0048] The specific steps are:
[0049] Weigh 0.0261g of the hapten (DM, 0.05mmol) into a 50ml small beaker, add four drops of absolute ethanol in an ice bath, and stir to dissolve. Then add 0.6ml of 1M hydrochloric acid and 0.4ml of 0.2M sodium nitrite to the solution, and stir well. Finally, 0.4 ml of DMF was added dropwise to the above homogeneous reaction solution.
[0050] Weigh 45mg of bovine serum albumin (BSA) or chicken egg serum albumin (OVA) into a 50ml small beaker, add a mixture of 5ml boric acid (0.2M) buffer solution (ph=8.7) and 1.2ml DMF to dissolve. Under ice-bath conditions, the active hapten solution was added dropwise to the stirring protein, and the dropwise addition was completed within 50 minutes. The reaction mixture was stirred in an ice bath to continue the reaction for 45 minutes, and was put into a...
Embodiment 3
[0051] Example 3 Determination of Antigen
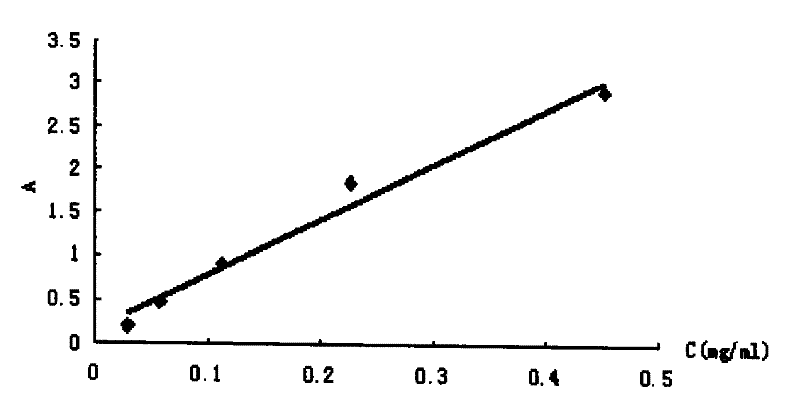
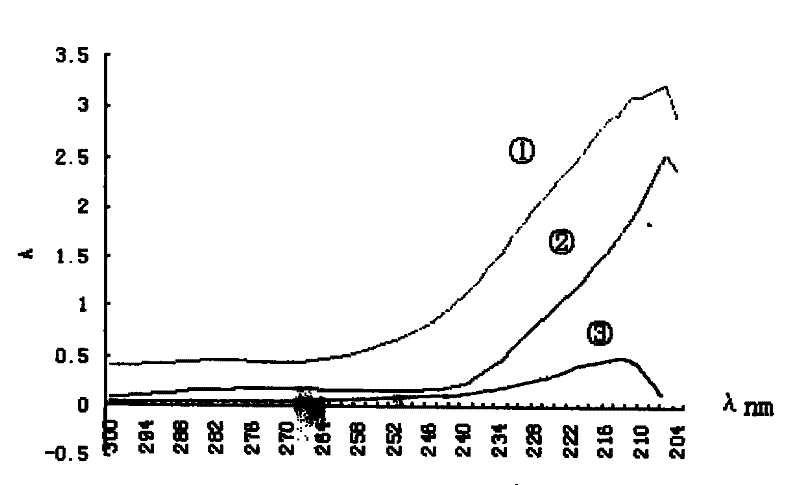
[0052] Weighed 21.7 mg of the synthesized deltamethrin hapten (DM), diluted it with absolute ethanol, prepared into series concentrations of 0.0283, 0.0567, 0.1131, 0.2263, and 0.4525 mg / ml, and carried out UV spectrum scanning respectively; Read the absorbance Amax at the maximum absorption peak of the hapten on the spectrogram, and use it as the DM concentration (C DM ) versus absorbance standard absorbance curve.
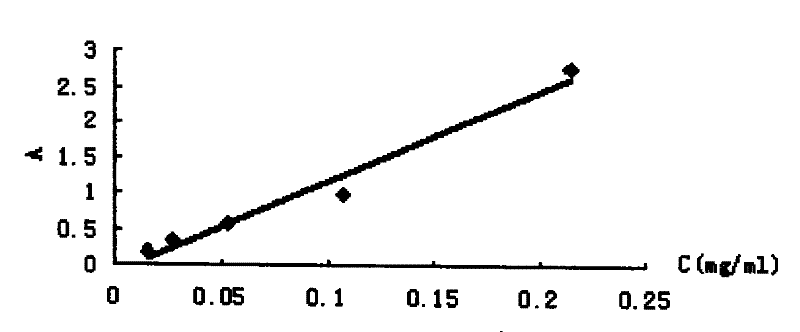
[0053] Weigh 1.28 mg of BSA, dilute it in phosphate buffer solution (PH=7.4), and make it into series concentrations of 0.015, 0.027, 0.053, 0.107, 0.214 mg / ml, and scan the UV spectrum respectively; read out from the UV spectrum of BSA Absorbance Amax at the maximum absorption peak of bovine serum albumin was used as BSA concentration (C BSA ) versus absorbance standard absorbance curve.
[0054] The dialyzed hapten and bovine serum albumin conjugate (DM-BSA) were tested by the Lowry method [10] The protein concent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com