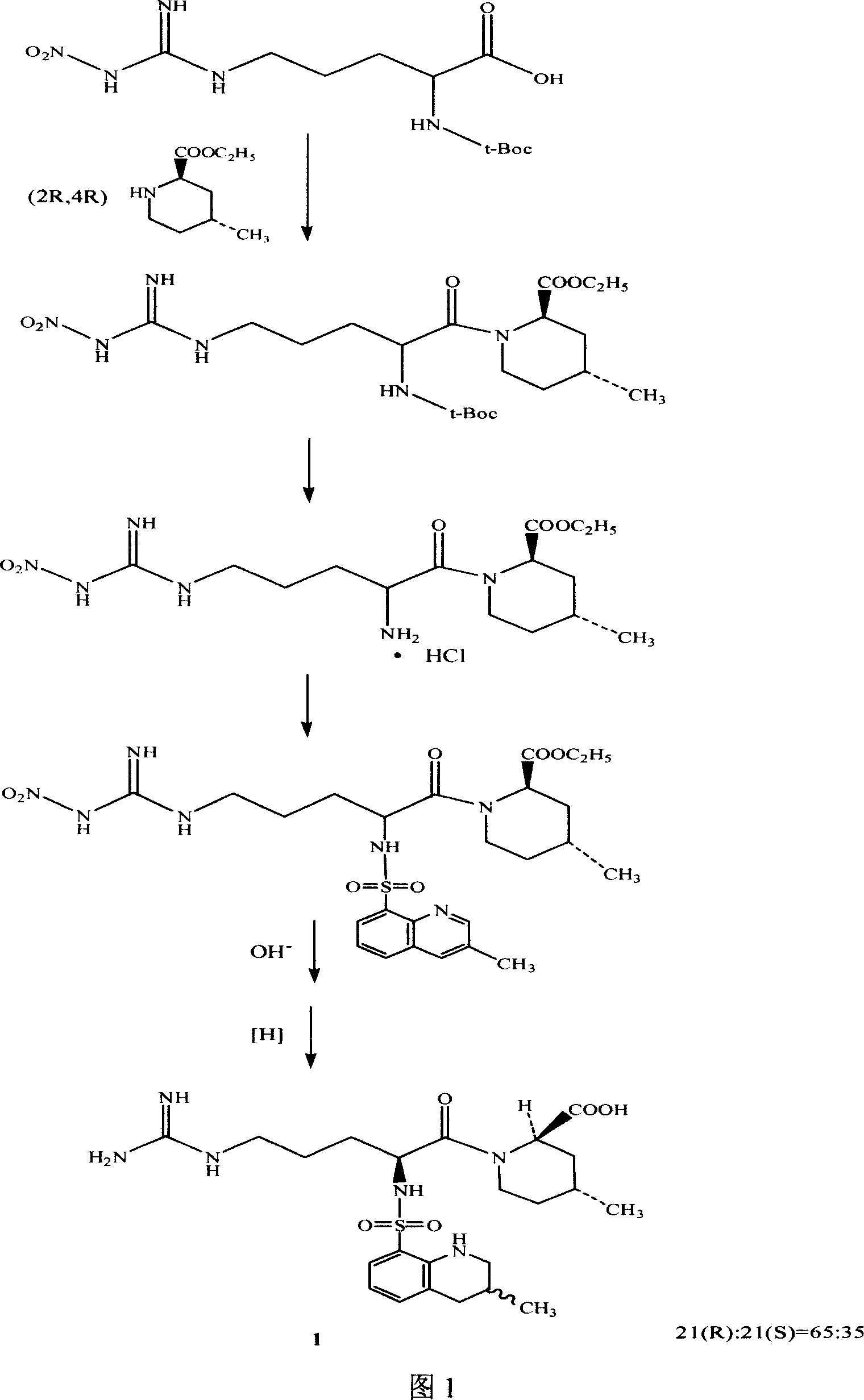
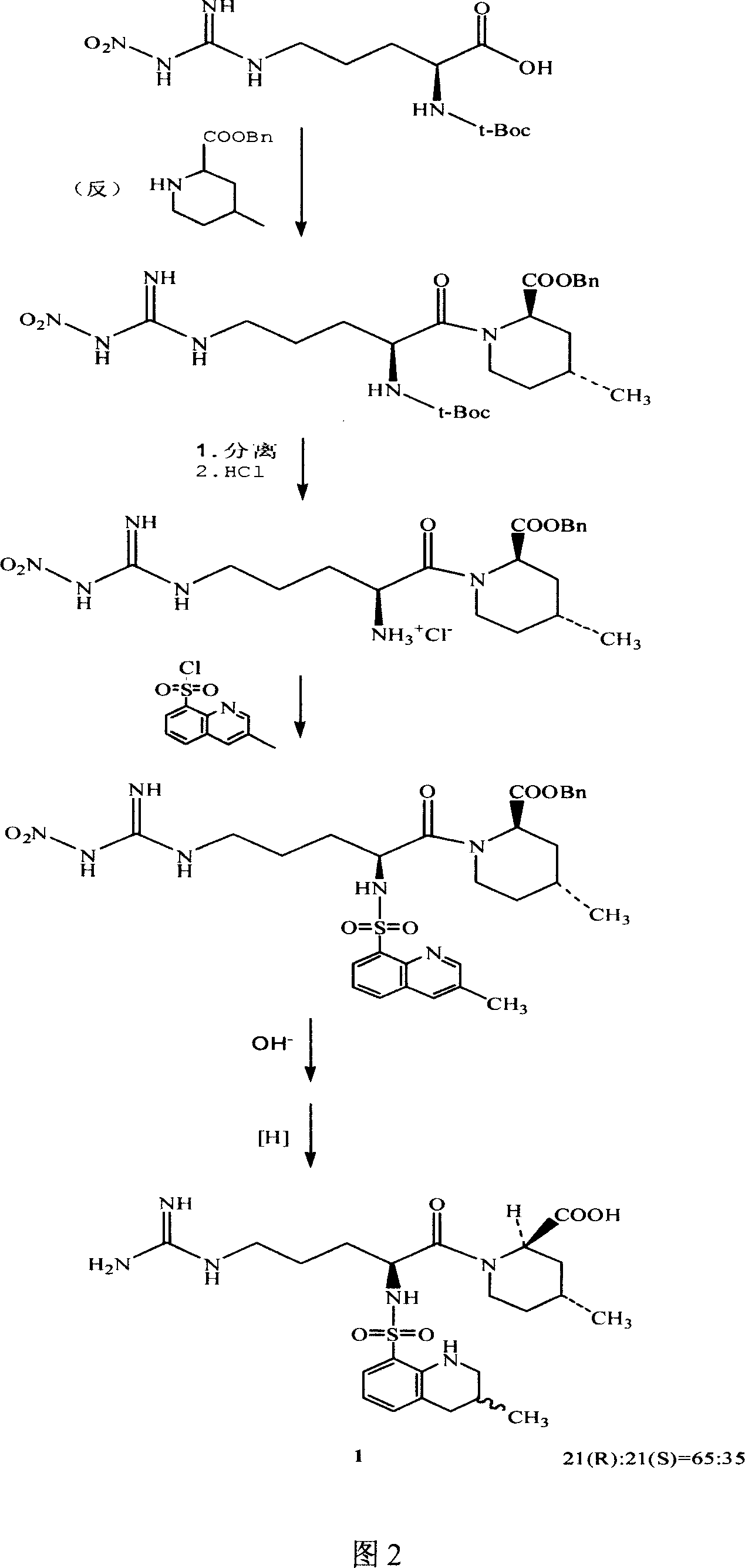
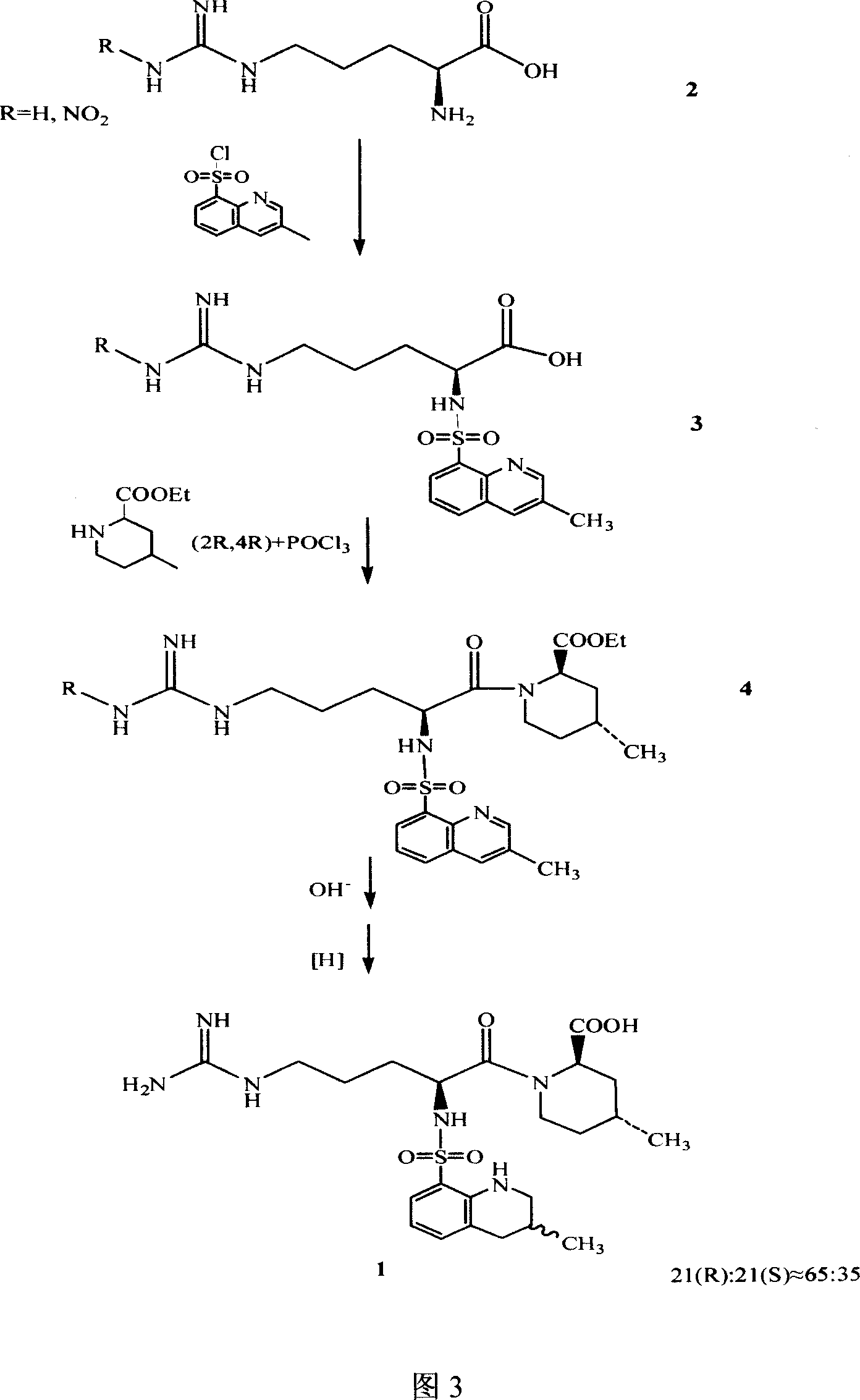
Argatroban separation method
A technology for the separation of argatroban and its separation method, which is applied in the field of separation of the optical isomer compound argatroban, and can solve problems such as unsuitability for industrial production, low yield, and failure to reach a practical level.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1. Add 7 grams of Argatroban (R: S=65: 35) to 210 ml of 85% aqueous methanol solution, heat to reflux for 6 hours until transparent, cool to room temperature, and leave to stand for 5 hours to separate out white crystals, filter, 80 ° C Drying under high temperature, weight 5.6 grams, 21 (S) content 42.5% (HPLC method).
Embodiment 2
[0035] Example 2. 5 grams of Argatroban (R: S=30: 70) was added to 100 ml of 50% aqueous methanol solution, heated to reflux for 5 hours until transparent, cooled to room temperature, left to stand for 4 hours, and white crystals were precipitated, filtered and dried , weight 2.5 g, 21(S) content 85.0% (HPLC method).
Embodiment 3
[0036]Embodiment 3. 2 grams of Argatroban (R: S=23: 76), was added in 60ml of 20% aqueous methanol solution, heated to reflux for 3 hours to transparent, cooled to room temperature, left to stand for 3 hours, precipitated solid, filtered, dried to obtain 0.9 g of white crystals, 21(S) content 94.9% (HPLC method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com