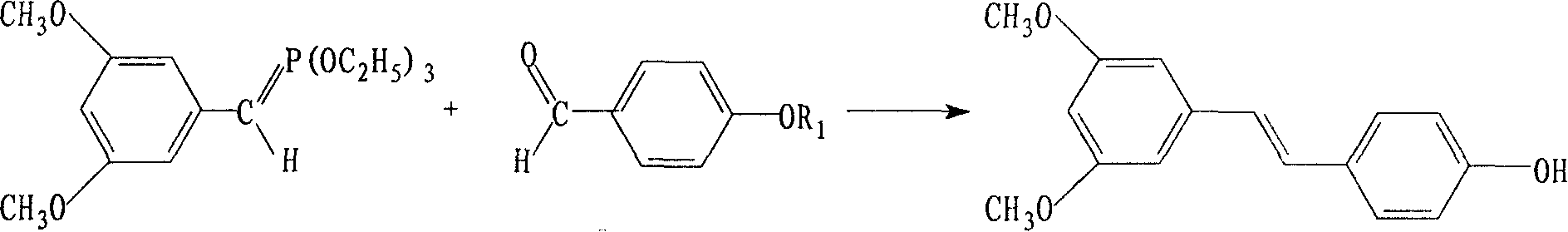
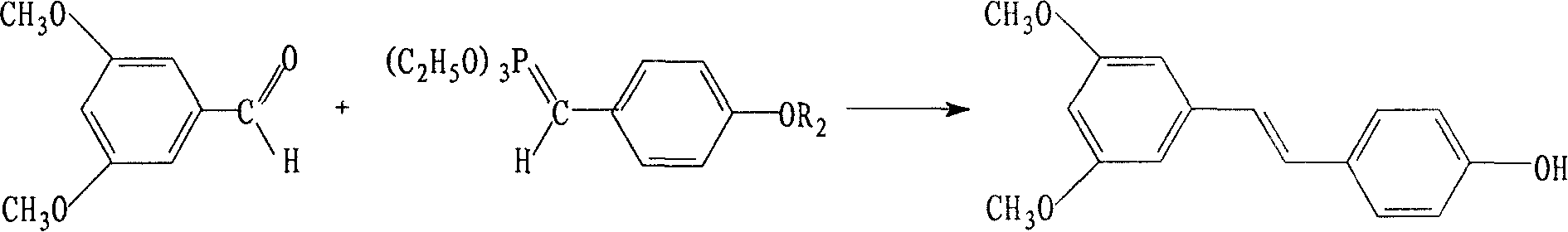
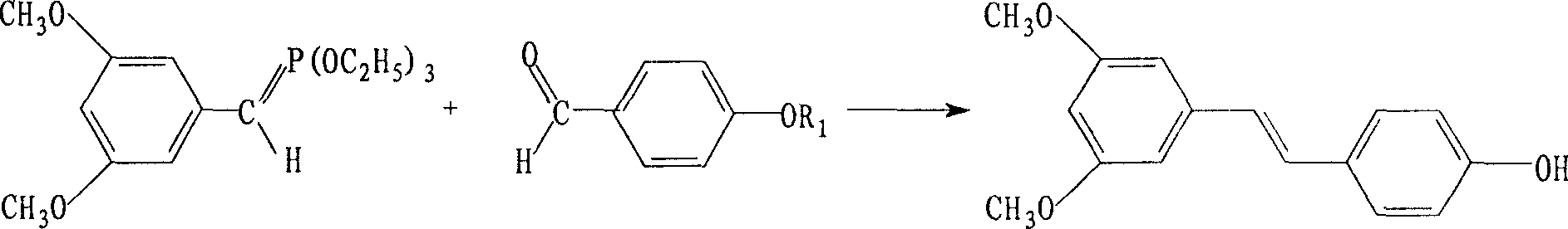
Synthetic method of pterostilbene
The technology of pterostilbene and compound is applied in the synthesis field of phytoestrogen pterostilbene, can solve the problems of low yield, high price, poor stereoselectivity and the like, and achieves the effects of high stereoselectivity, simple process and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Step A: Dissolve 11.7g (0.11mol) of p-hydroxybenzaldehyde in 100ml of dichloromethane, add 17.4ml (0.33mol) of N,N-diisopropylethylamine, then add dropwise 18.8ml of chloromethyl methyl ether ( 0.22mol), refluxed for 3.5 hours, evaporated the solvent, added 200ml of ethyl acetate and 200ml of water. Ethyl acetate was fractionated three times, washed with saturated NaCl, dried, and the solvent was evaporated to obtain crude methoxybenzaldehyde, which was used in the next reaction.
[0020] Step B: Add 21.8 g (0.11 mol) of 3,5-dimethoxybenzyl chloride and 39 g (0.24 mol) of triethyl phosphite into the reactor, keep the reaction at 140°C for 5 hours, and vacuum the excess at 105°C The triethyl phosphite was distilled off. Add dry DMF120ml, then add 12.5950% sodium methoxide under cooling, react at 5°C for 2 hours until dissolved, then add p-methoxymethoxybenzaldehyde into the reactor, react at room temperature for 2 hours, heat up to 100°C, gradually Cool down to room te...
Embodiment 2
[0023] Step A: Use 16.3g (0.12mol) of p-hydroxybenzyl alcohol and 15.2g (0.12mol) of benzyl chloride to condense into ether, then feed chlorine gas to obtain 23.4g (0.1mol) of 4'-benzyloxybenzyl chloride, add 39g (0.24mol) of triethyl phosphite was reacted at 140°C for 6 hours; 120ml of DMF and 12.5g of 50% sodium methoxide were added under cooling and reacted at 5°C for 2 hours until dissolved.
[0024] Step B: Add 17.4 g (0.1 mol) of 3,5-dimethoxybenzaldehyde to the above solution, react at room temperature for 1.5 hours, then raise the temperature to 100°C for 1.5 hours, and react at room temperature for 10 hours to obtain benzyl ether The compound crude product 33g was directly used in the next reaction.
[0025] Step C: Add 100ml of N,N-dimethylaniline and 33ml of dichloromethane to the above benzyl ether compound, stir at 0°C for 10 minutes, add 40g of anhydrous aluminum chloride, react for 6 hours, add 1.0M hydrochloric acid 20ml, stirred, the mixture was extracted wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com