Protein adsorption-preventing ocular lens material and method for producing same
A manufacturing method and protein technology, which can be used in glasses/goggles, intraocular lens, eye implants, etc., can solve the problem that the introduction reaction of phosphocholine bases cannot be fully carried out, does not show the effect of preventing protein adsorption, and is extremely difficult to obtain high yields. problems such as rate and high purity, to achieve excellent antifouling effect, improve wearing feeling, easy to control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
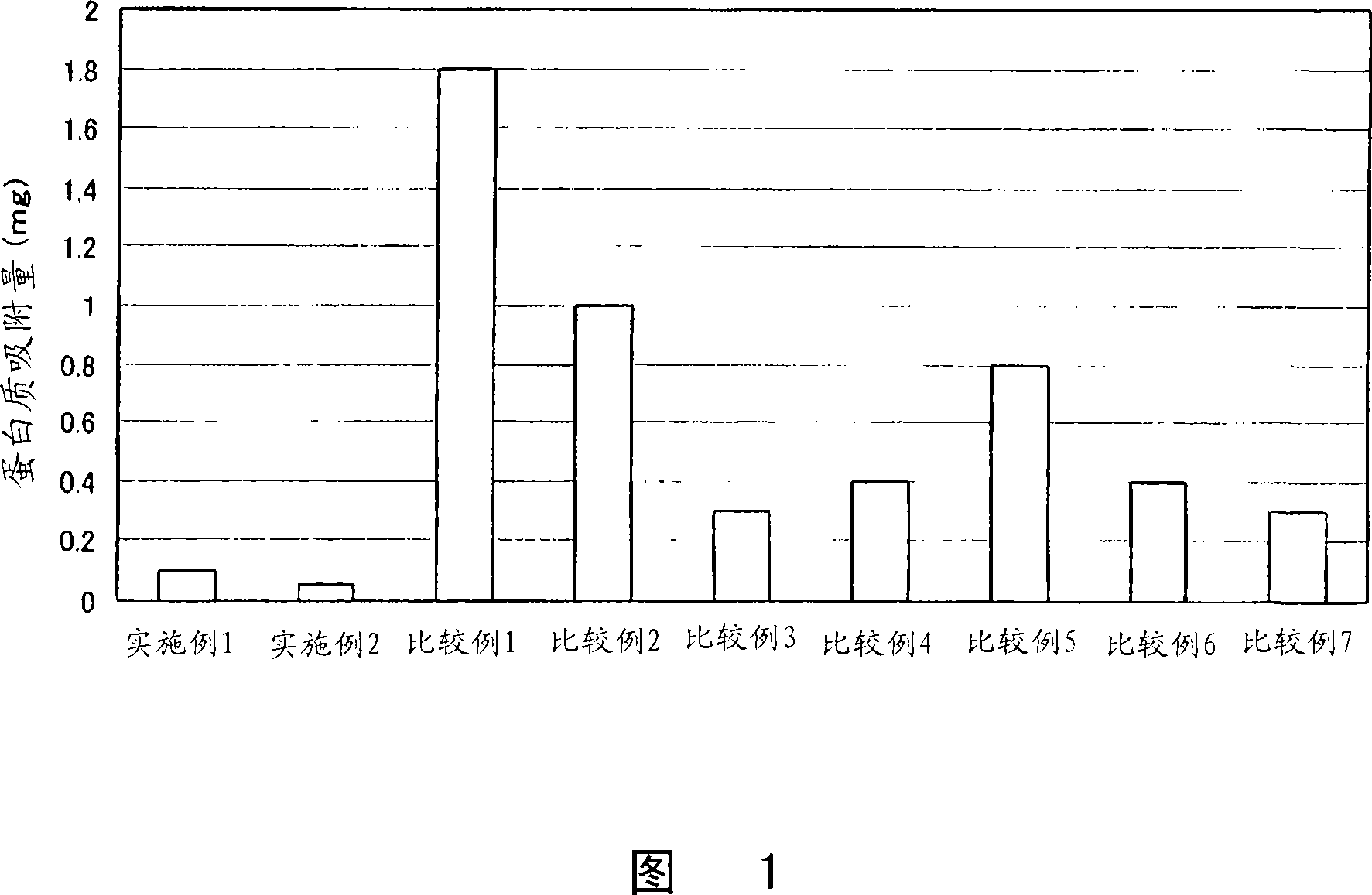
Examples
Embodiment 1
[0108] "Example 1"
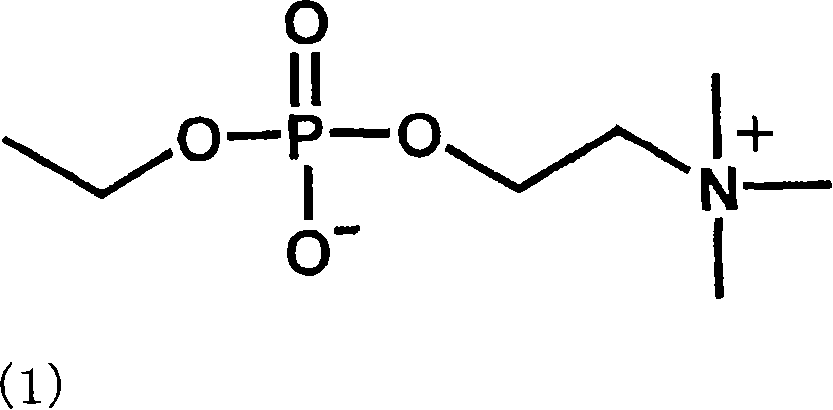
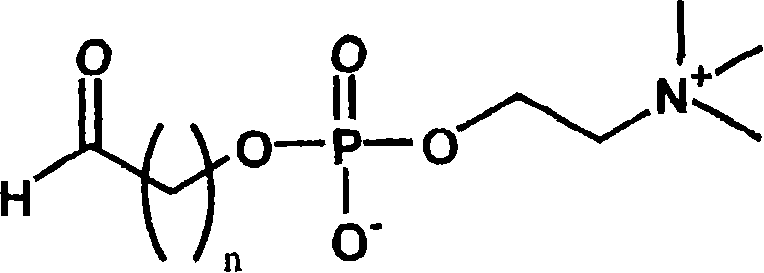
[0109]One piece of Polymacon was soaked in 2 ml of water, and 10 mg of the phosphorylcholine-containing compound of formula (2) was dissolved. Next, 2ml of 2N hydrochloric acid was added to adjust the concentration of the mixed solution to 1M hydrochloric acid, and then reacted at 70° C. for 5 hours. The reaction solution was cooled to room temperature, and washed sufficiently with pure water to obtain the target contact lens. The introduced amount of the phosphorylcholine group of the formula (1) was 0.0734 mol / mg.
Embodiment 2
[0110] "Example 2"
[0111] One tablet of Nelfilcon A was soaked in 2 ml of water, and 10 mg of the phosphorylcholine-containing compound of formula (2) was dissolved. Next, 2ml of 2N hydrochloric acid was added to adjust the concentration of the mixed solution to 1M hydrochloric acid, and then reacted at 40° C. for 5 hours. The reaction solution was cooled to room temperature, and washed sufficiently with pure water to obtain the target contact lens. The introduced amount of the phosphorylcholine group of the formula (1) was 0.1688 μmol / mg.
[0112] "Method for quantifying phosphorylcholine groups of formula (1)"
[0113] The obtained contact lens is immersed in perchloric acid and heated to 180°C to decompose. The obtained solution was diluted with water, heptamolybdate hexaammonium tetrahydrate and L ascorbic acid were added thereto, the color was developed at 95°C for 5 minutes, and the amount introduced was determined by measuring the absorbance at 710 nm. The calibra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com