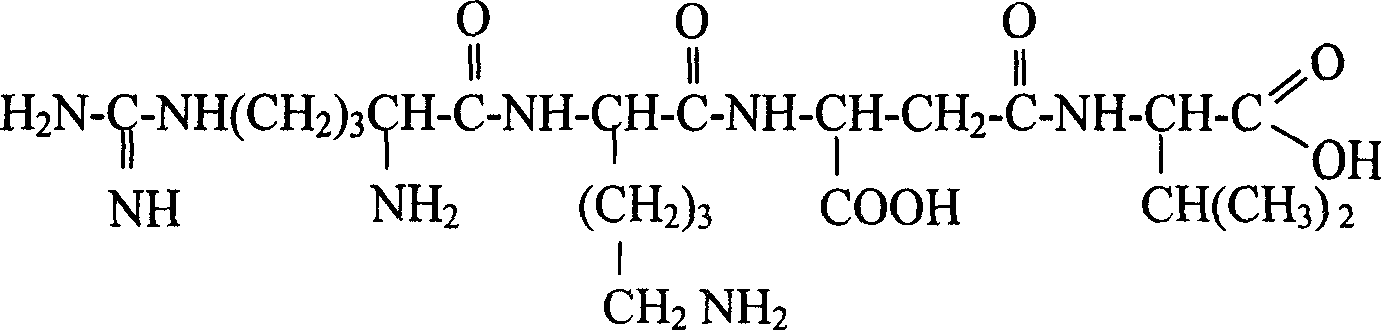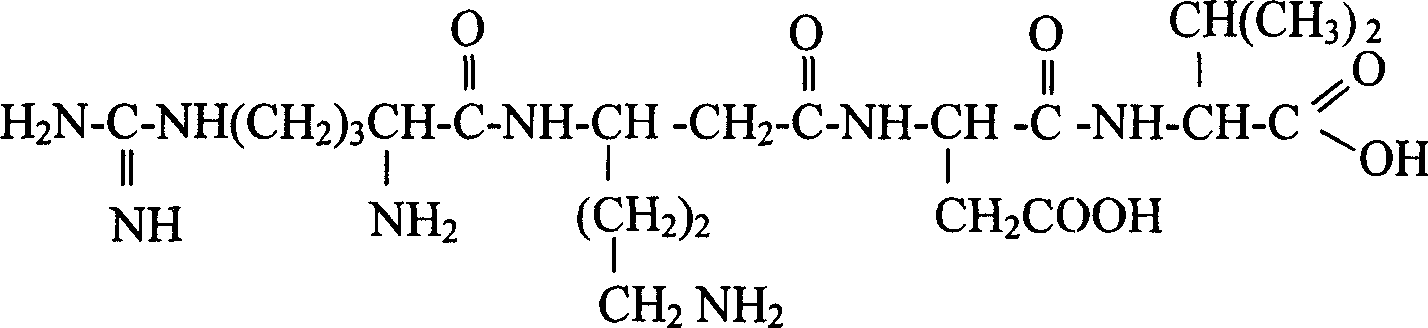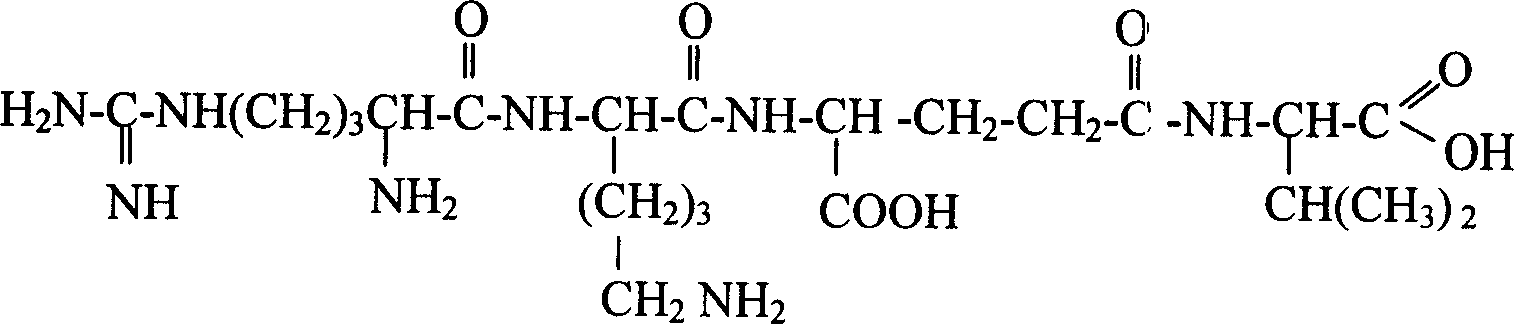Thymus tetrapeptide active isomer and its preparation method and medicinal uses
An isomer and thymus technology, which is used in the preparation of immunomodulatory drugs, anti-tumor drugs and anti-oxidant drugs, can solve problems such as unfavorable treatment and short half-life, and achieves improved bioavailability, improved stability, prolonged In vivo half-life effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Solid Phase Synthesis of LW401
[0112] LW401: Arg-Lys-β-Asp-Val
[0113]
[0114] Raw materials: Fmoc-Val-Wang resin, Fmoc-β-Asp-otBu, Fmoc-Arg(pbf)-OH, Fmoc-Lys(Boc)-OH, coupling reaction condensing agent is benzotriazole-1-oxygen- Tris(dimethylamino)phosphorus hexafluorophosphate (BOP), 1-hydroxybenzotriazole (HOBT), N-methylmorpholine (NMM). The deprotecting agent is 20% piperidine / DMF solution. The cleavage reagent is trifluoroacetic acid.
[0115] Reaction: Weigh Fmoc-Val-Wang resin, swell in DMF at room temperature for 30 minutes, and wash with DMF three times. Add 20% piperidine / DMF solution and react at room temperature for 1 hour to deprotect. Then wash with DMF three times, Fmoc-β-Asp-otBu, react at room temperature for 3 hours, then wash with DMF three times, add 20% piperidine / DMF solution, react at room temperature for 1 hour to deprotect. Then wash with DMF three times, add Fmoc-Lys(Boc)-OH, react at room temperature for 3 hours, then wash with DM...
Embodiment 2
[0119] Solid Phase Synthesis of LW402
[0120] LW402: Arg-β-Lys-Asp-Val
[0121]
[0122] Raw materials: Fmoc-Val-Wang resin, Fmoc AspotBu, Fmoc-Arg(pbf)-OH, Fmoc-β-Lys(Boc)-OH, coupling reaction condensing agent is benzotriazole-1-oxygen-tri( Dimethylamino)phosphorus hexafluorophosphate (BOP), 1-hydroxybenzotriazole (HOBT), N-methylmorpholine (NMM). The deprotecting agent is 20% piperidine / DMF solution. The cleavage reagent is trifluoroacetic acid.
[0123] Reaction: Weigh Fmoc-Val-Wang resin, swell in DMF at room temperature for 30 minutes, and wash with DMF three times. Add 20% piperidine / DMF solution and react at room temperature for 1 hour to deprotect. Then wash with DMF three times, Fmoc-Asp-otBu, react at room temperature for 3 hours, then wash with DMF three times, add 20% piperidine / DMF solution, react at room temperature for 1 hour to deprotect. Then wash with DMF three times, add Fmoc-β-Lys(Boc)-OH, react at room temperature for 3 hours, then wash with DMF ...
Embodiment 3
[0127] Solid Phase Synthesis of LW403
[0128] LW403: Arg-Lys-γ-Glu-Val
[0129]
[0130] Raw materials: Fmoc-Val-Wang resin, Fmoc-Lys-(Boc)-OH, Fmoc-Arg(pbf)-OH, Fmoc-γ-Glu-otBu, coupling reaction condensing agent is benzotriazole-1-oxygen - Tris(dimethylamino)phosphorus hexafluorophosphate (BOP), 1-hydroxybenzotriazole (HOBT), N-methylmorpholine (NMM). The deprotecting agent is 20% piperidine / DMF solution. The cleavage reagent is trifluoroacetic acid.
[0131] Reaction: Weigh Fmoc-Val-Wang resin, swell in DMF at room temperature for 30 minutes, and wash with DMF three times. Add 20% piperidine / DMF solution and react at room temperature for 1 hour to deprotect. Then wash with DMF three times, add Fmoc-γ-Glu-otBu, react at room temperature for 3 hours, then wash with DMF three times, add 20% piperidine / DMF solution, react at room temperature for 1 hour to deprotect. Then wash with DMF three times, add Fmoc-Lys(Boc)-OH, react at room temperature for 3 hours, then wash w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com