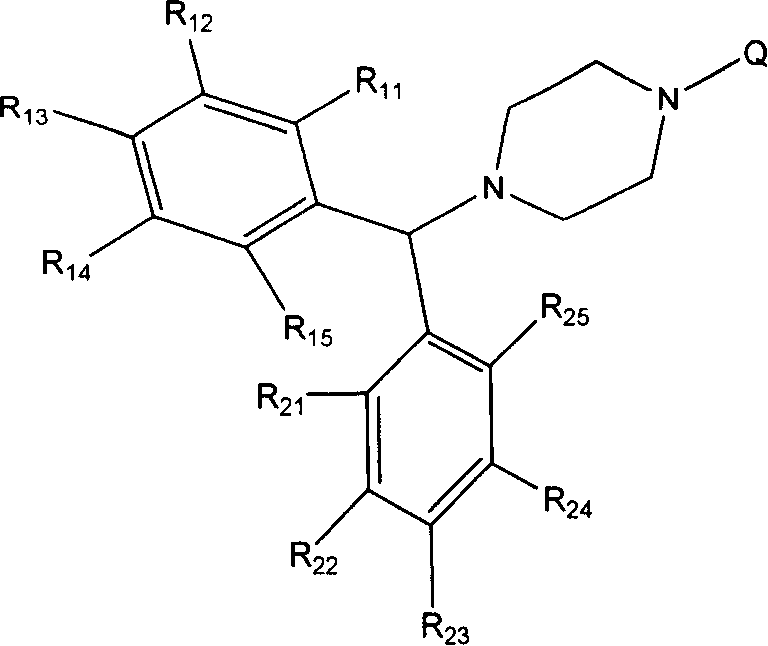
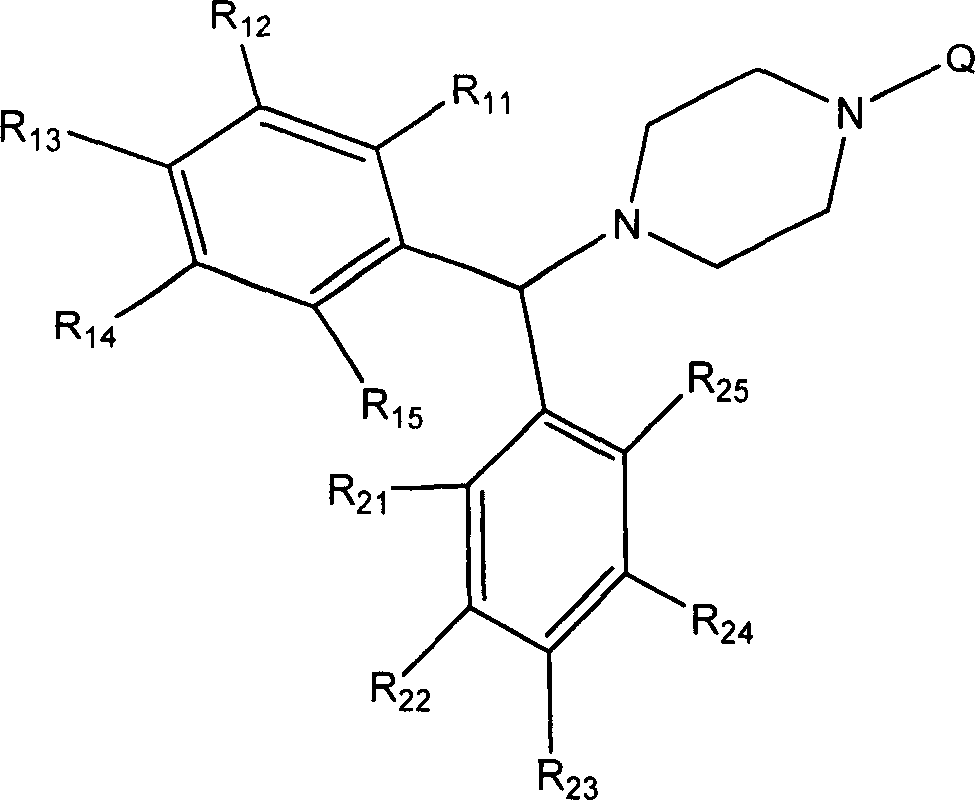
3CL protease inhibitor of non-peptide SARS coronavirus and use thereof
A coronavirus, protease technology, applied in antiviral agents, respiratory diseases, medical preparations containing active ingredients, etc., to achieve high accuracy and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1. The synthesis of oxamide
[0029] [References: Liu Xiang, Liu Tinghui, Liu Baili. Journal of Shenyang Pharmaceutical University, 2001, 18(5), 327-328. ]
[0030] 1. Synthetic route:
[0031]
[0032] 2. Experimental steps:
[0033] Preparation of A, 1-isopropenyl-1,3-dihydro-2H-benzimidazol-2-one (2)
[0034] In a reaction flask equipped with a water separator, add 54g (0.5mol) of o-phenylenediamine and 220ml of xylene, and after heating and stirring to reflux, slowly add a mixture of 71.5g (0.55mol) of ethyl acetoacetate and 20ml of xylene , After dropping, stir and reflux, azeotropic dehydration until no water drops appear, continue to stir and reflux for 2h. After the reaction is complete, cool and precipitate crystals, filter and dry to obtain 56.2 g of white granular crystals (3) (64.6% yield), mp 121-122°C
[0035] B, the preparation of 1-(3-chloropropyl)-1,3-dihydro-2H-benzimidazol-2-one (3)
[0036] In the reaction flask, add (2) 5.042g (...
Embodiment 2
[0044] The synthesis of embodiment 2.1-benzhydryl-4-benzylpiperazine (pkumdl_cvs_1006)
[0045] According to the operating conditions of step E in Example 3, (4) was replaced by benzyl chloride to obtain 1-benzhydryl 4-benzylpiperazine, which was recrystallized from acetone to obtain colorless crystals with a yield of 81.2%.
[0046] 1 HNMR (CDCl 3 )δ: 2.46(s, 8H, hydrogen on the piperazine ring), 3.51(s, 2H, CH 2 Ph), 4.23 (s, 1H, ArCH), 7.14-7.42 (m, 15H, hydrogen on the benzene ring)
Embodiment 3
[0047] The synthesis of embodiment 3.1-benzhydryl-4-[(pyridin-3-yl) methyl] piperazine (pkumdl_cvs_1007)
[0048] According to the operating conditions of step E in Example 3, (4) is replaced by 3-chloromethylpyridine hydrochloride to obtain 1-benzhydryl-4-[(pyridin-3-yl)methyl]piperazine , recrystallized from acetone to obtain colorless crystals with a yield of 61.8%.
[0049] 1 HNMR (CDCl 3 )δ: 2.41 (s, 8H, hydrogen on the piperazine ring), 3.49 (s, 2H, CH 2 -), 4.27 (s, 1H, CH-), 7.12-7.42, 7.65-7.69, 8.43-8.45 (m, d, d, 14H, hydrogen on benzene ring and pyridine ring).
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com