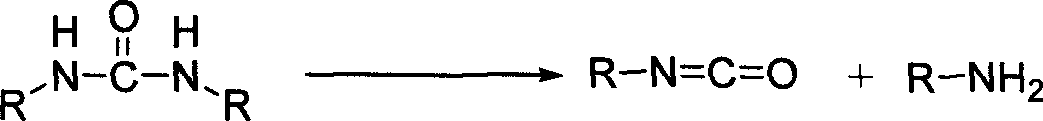
Process for preparing disubstituted urea
A technology for disubstituted urea and urea, which is applied in the preparation of urea derivatives, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of polluting the environment waste gas-hydrogen chloride and hydrogen halide pollute the environment, and the equipment is corroded. The effect of decolorization treatment, simple post-treatment process and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Mix urea and aniline compounds in a ratio of 1:1, add them into a container containing N,N-dimethylformamide as a solvent, raise the temperature to 150°C while stirring, and keep the reaction time 120 minutes, then the reaction system was cooled to room temperature. After adding water to the reaction system, the white solid obtained by suction filtration is the corresponding urea. The filtrate passes through the separation, and the reaction substrate can be put back into use. Diphenylurea was dried and weighed, and the yield was 20%.
Embodiment 2
[0024] Same as Example 1, the ratio of urea to aniline is 1:4, and the yield of diphenylurea is 40%.
Embodiment 3
[0026] With example 2, temperature is 160 ℃, and the yield of diphenylurea is 56%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com