Method for synthesizing cyclopentadiene polymer
A cyclopentadiene and polymer technology, applied in the field of cyclopentadiene polymer synthesis, can solve the problem of low number-average molecular weight of cyclopentadiene homopolymers and copolymers, and little research on the detailed mechanism of co-catalysts , The research on the synthesis of new cyclopentadiene copolymers and the research on their properties are few problems, so as to achieve the effect of single component and clear structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
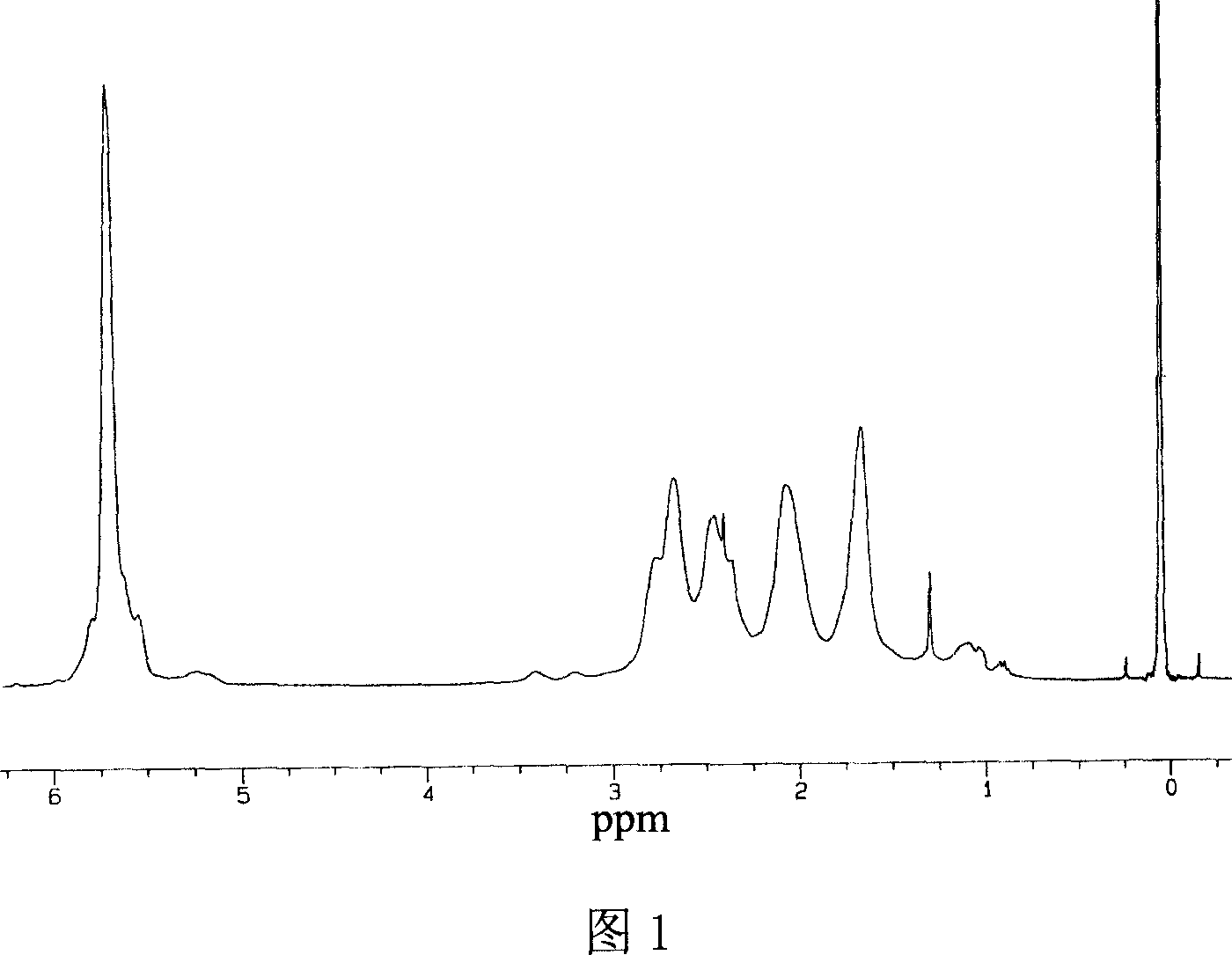
[0023] Replace the fully dried 100 ml polymerization reaction bottle with nitrogen twice under normal pressure, maintain the nitrogen atmosphere, add 0.05 moles of cyclopentadiene monomer, add 0.005 moles of methyl aluminoxane at -84 ° C, and keep - 84 DEG C reacted for 10 minutes, then added 20 milliliters of concentration and was 5% hydrochloric acid ethanol solution to terminate the reaction, and the polymer yield was 77%, and only contained 1,2- and 1, two kinds of chain structural units of 1,4-structure in the polymer, Wherein the 1,2-structure content is 50% ( 1 HNMR spectrogram analysis results, see accompanying drawing 1), number average molecular weight 32000.
Embodiment 2
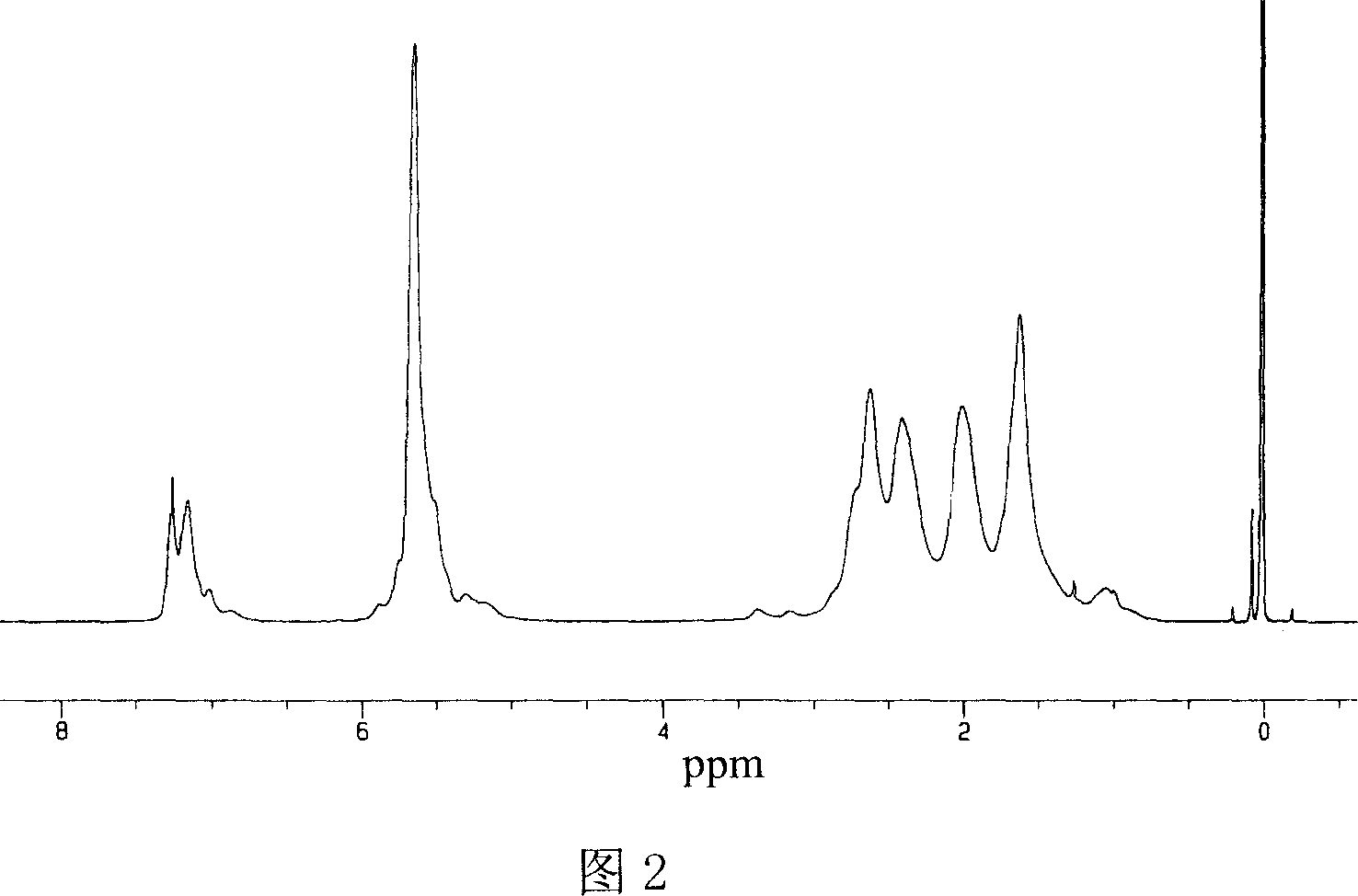
[0025] Replace the fully dried 100 ml polymerization reaction bottle with nitrogen twice under normal pressure, maintain the nitrogen atmosphere, add 0.05 moles of cyclopentadiene monomer, add 0.005 moles of methyl aluminoxane at -84 ° C, and keep - 84 DEG C reacted for 12 hours, then added 20 milliliters of concentration and was 5% hydrochloric acid ethanol solution to terminate the reaction, and the polymer yield was 97%, and only contained 1,2- and 1, two kinds of chain structural units of 1,4-structure in the polymer, Among them, the 1,2-structure content is 57%, and the number average molecular weight is 43000.
Embodiment 3
[0027] Replace the fully dried 100 ml polymerization reaction bottle with nitrogen twice under normal pressure, maintain the nitrogen atmosphere, add 0.1 mole of cyclopentadiene monomer, 15 ml of toluene, and add 0.01 mole of methyl aluminoxane at 15 °C , keep the reaction at 15°C for 7 hours, then add 20 milliliters of 5% ethanol hydrochloric acid solution to terminate the reaction, the polymer yield is 45%, and the polymer only contains two chain structures of 1,2- and 1,4-structure unit, wherein the 1,2-structure content is 40%, and the number average molecular weight is 31000.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com