Quality control method of honeysuckle, scutellarta root and extract thereof and formulation containing the extract
A quality control method, honeysuckle extract technology, applied to medical preparations containing active ingredients, measuring devices, drug combinations, etc., can solve problems such as correlation studies that have not been reported, to ensure the safety of use and control stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
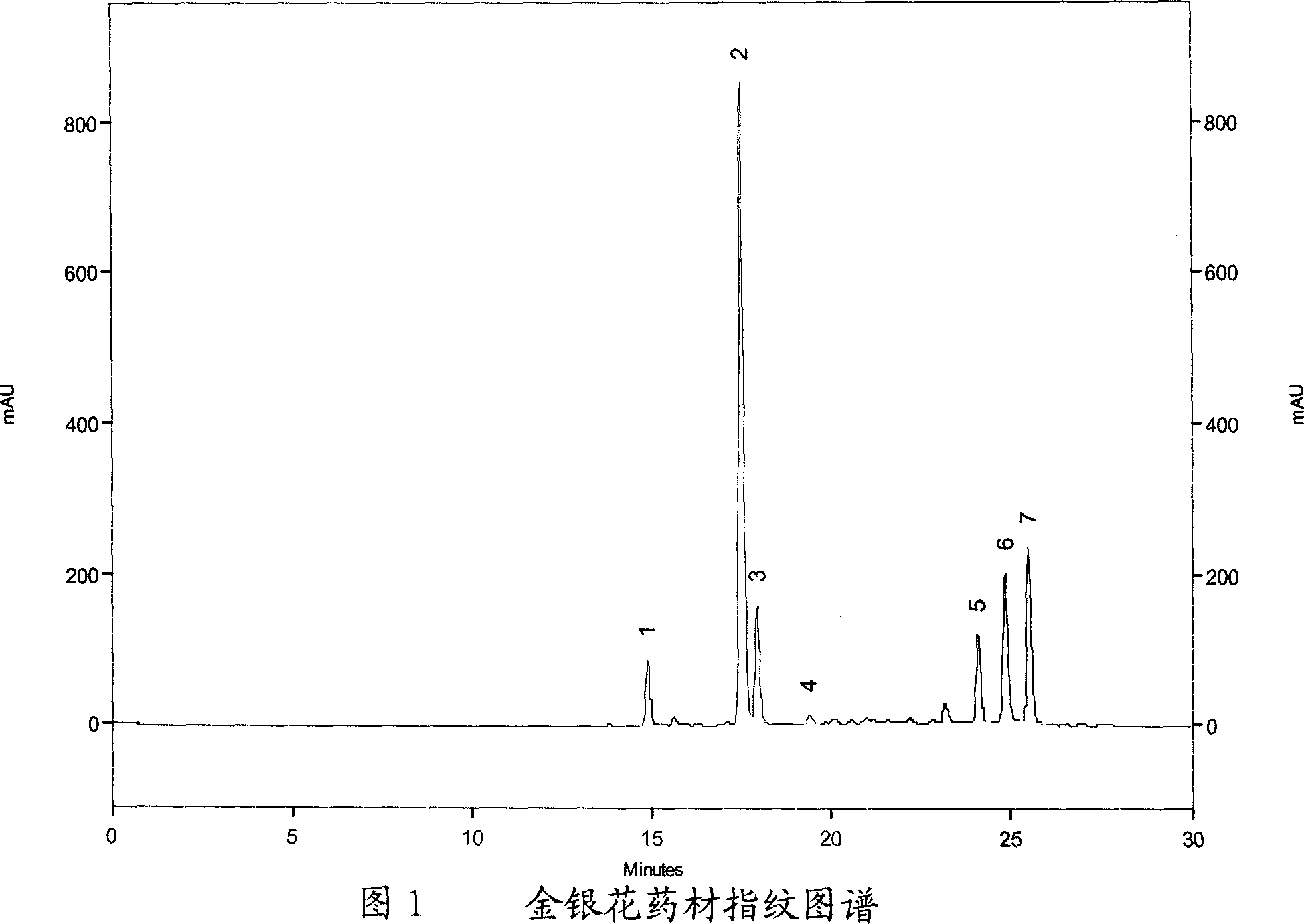
[0053] Embodiment one, honeysuckle medical material fingerprint collection
[0054] 1. Chromatographic conditions
[0055] laballiance high performance liquid chromatography; seriesIII binary pump; UV6000LP detector; Ezchrom Elite workstation.
[0056] Chlorogenic acid reference substance (provided by China National Institute of Pharmaceutical and Biological Products, batch number: 11075-200212); acetonitrile is chromatographically pure, and other reagents are analytically pure.
[0057] Chromatographic column: Kromasil C 18 (Ф4.6×250mm, 5um); mobile phase A: acetonitrile, mobile phase B: 0.1% phosphoric acid; using gradient elution, the elution procedure is shown in the table below; detection wavelength: 327nm; flow rate: 1.0ml / min; column temperature : 30°C; injection volume: 10ul.
[0058] time (min)
A(%)
B(%)
0
5
25
30
5
5
35
5
95
95
65
95
[0059] 2. Preparat...
Embodiment 2
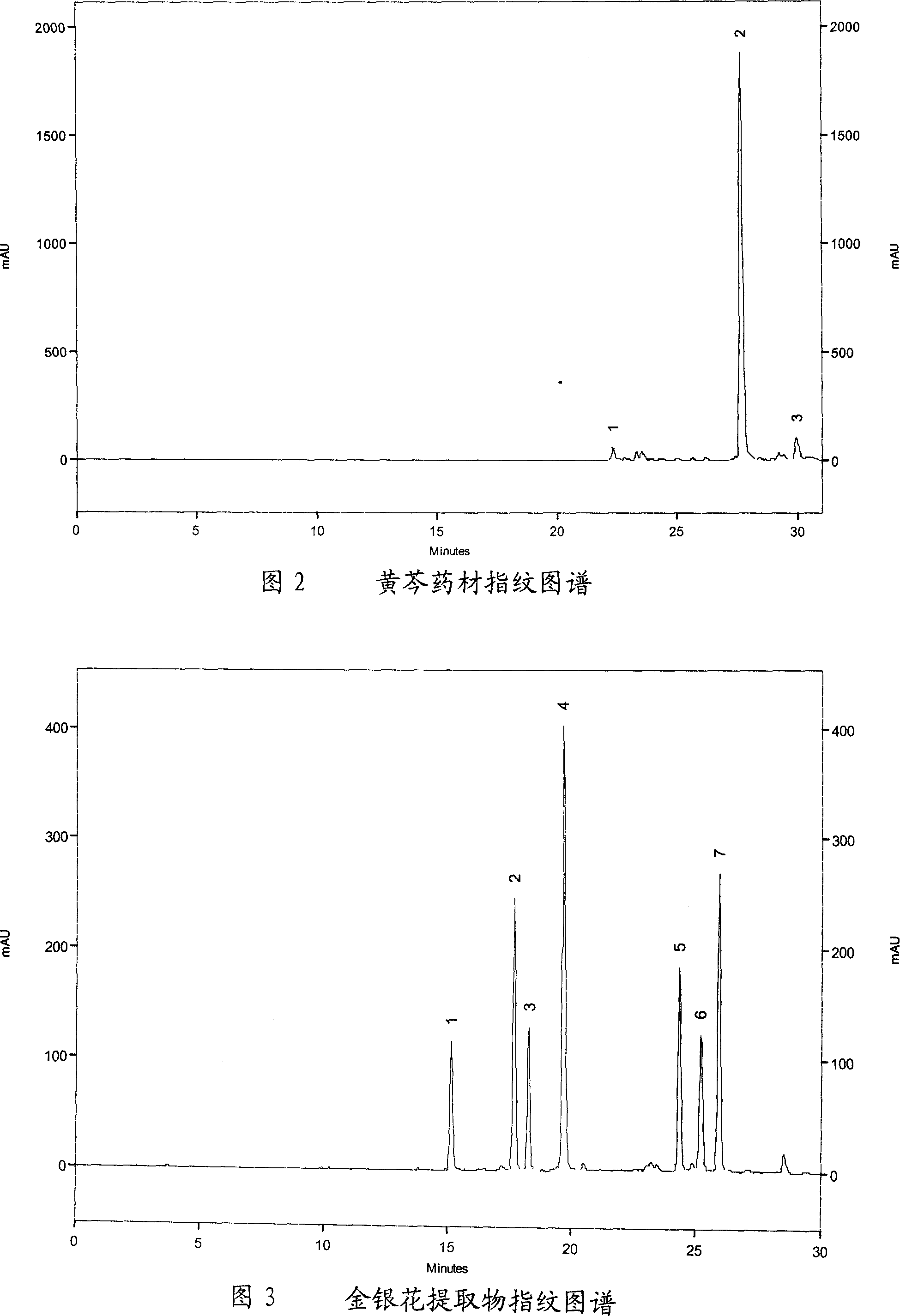
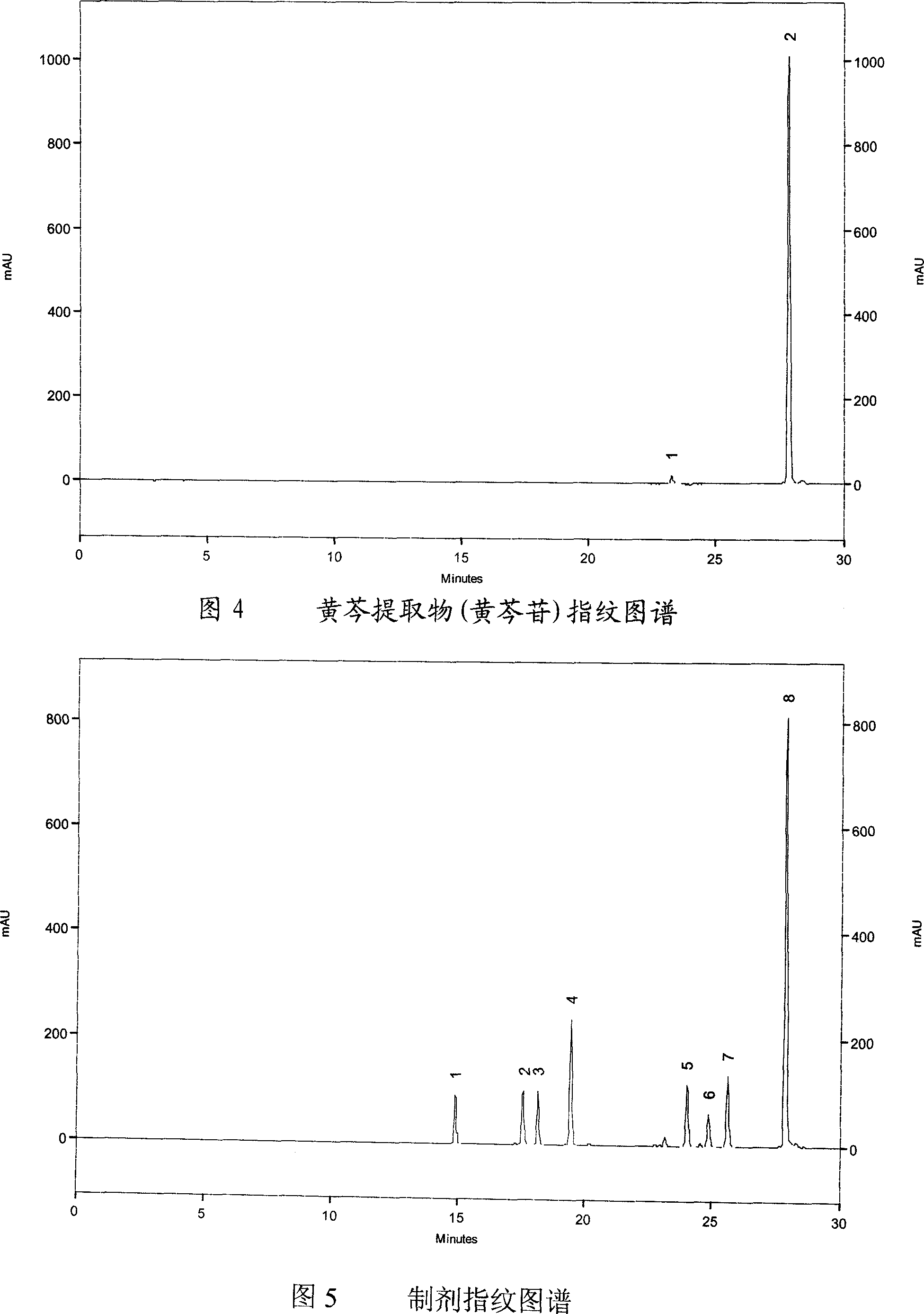
[0074] Embodiment two, honeysuckle extract fingerprint
[0075] 1. Chromatographic conditions
[0076] laballiance high performance liquid chromatography; seriesIII binary pump; UV6000LP detector; Ezchrom Elite workstation.
[0077] Chlorogenic acid reference substance (provided by China National Institute of Pharmaceutical and Biological Products, batch number: 11075-200212); honeysuckle extract (self-made); acetonitrile is chromatographically pure, and the rest of the reagents are analytically pure.
[0078] Column: Kromasil C 18 (Ф4.6×250mm, 5um); mobile phase A: acetonitrile, mobile phase B: 0.1% phosphoric acid; using gradient elution, the elution procedure is shown in the table below; detection wavelength: 327nm; flow rate: 1.0ml / min; column temperature : 30°C; injection volume: 10ul.
[0079] time (min)
[0080] 2. Preparation of reference substance solution: Take 5 mg of chlorogenic acid reference substance, weigh it accurately, put it in a 100ml volumetri...
Embodiment 3
[0089] Embodiment three, Scutellaria baicalensis medicinal material fingerprint
[0090] 1. Chromatographic conditions
[0091]High-performance liquid chromatography: lab alliance seriesIII binary pump; UV6000LP detector; Ezchrom Elite workstation.
[0092] Baicalin (provided by China National Institute of Pharmaceutical and Biological Products, batch number: 11015-200212); acetonitrile was chromatographically pure, and the rest of the reagents were analytically pure.
[0093] Column: Kromasil C 18 (Ф4.6×250mm, 5um); mobile phase A: acetonitrile, mobile phase B: 0.1% phosphoric acid; using gradient elution, the elution procedure is shown in the table below; detection wavelength: 327nm; flow rate: 1.0ml / min; column temperature : 30°C; injection volume: 10ul.
[0094] time (min)
[0095] 2. Preparation of reference substance solution: Take 6 mg of baicalin reference substance, weigh it accurately, put it in a 100ml volumetric flask, add methanol to dissolve and dilu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com