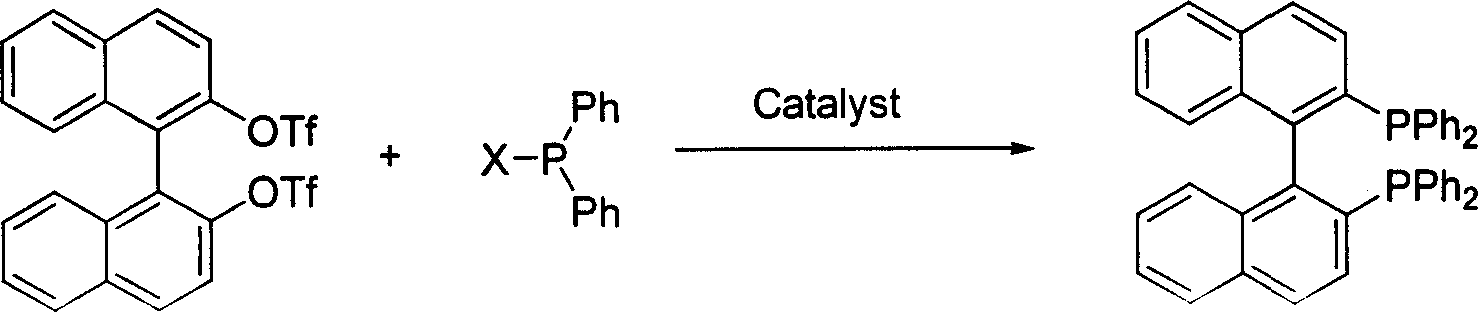
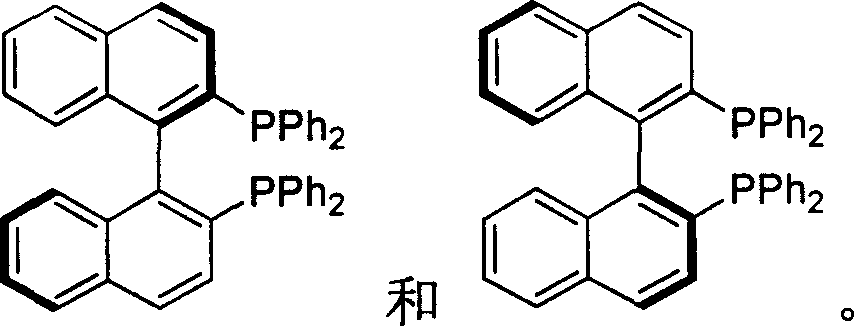
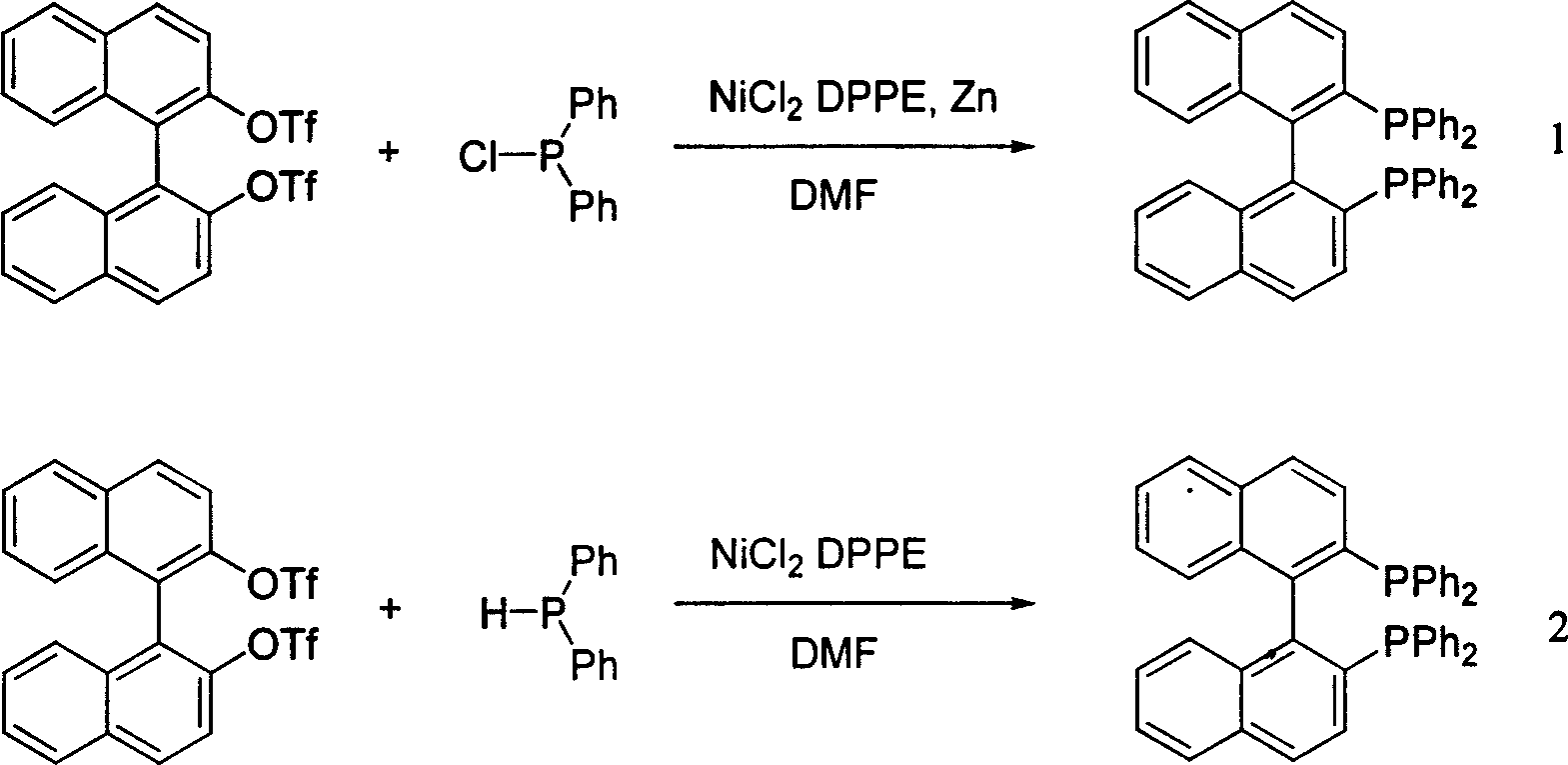
Synthesis of chiral 2,2'-bi(diphenyl phosphine)-1,1'-binaphthalene
A technology of diphenylphosphine and a synthesis method, which is applied in the field of one-step synthesis of chirality, can solve the problems of containing impurities and low yield, and achieves the effects of improved yield, simple purification method, and suitability for large-scale industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 1.1 g BINOLOTf (2 mmol) and 0.064 g NiCl into a 20 ml reaction flask. 2 (dppe), 0.885 g (4 mmol) of diphenylphosphonium chloride, 5 ml of anhydrous DMF, and then 0.602 g of co-catalyst Cu powder. The reaction solution was heated to 100°C for 24 hours. The color analysis of the thin-layer silica gel plate shows that the raw materials have reacted completely. The reaction solution was cooled to room temperature and diluted with 20 ml of dichloromethane. The reaction solution was washed successively with 5% dilute hydrochloric acid, water, and saturated aqueous sodium bicarbonate solution, then separated, dried with anhydrous sodium sulfate, filtered to remove the desiccant and spin dried. After purification by silica gel chromatography, 1.05 g of pure product was obtained. The yield was 84%.
Embodiment 2
[0034] Add 2.2 g BINOLOTf (4 mmol) and 0.12 g NiCl into a 20 ml reaction flask. 2(dppe), 1.77 g (4 mmol) of diphenylphosphonium chloride, 10 ml of anhydrous DMF, and then 0.60 g of co-catalyst iron powder was added to it. The reaction solution was heated to 100°C for 24 hours. The color analysis of the thin-layer silica gel plate shows that the raw materials have reacted completely. The reaction solution was cooled to room temperature and diluted with 40 ml of dichloromethane. The reaction solution was washed successively with 5% dilute hydrochloric acid, water, and saturated aqueous sodium bicarbonate solution, then separated, dried with anhydrous sodium sulfate, filtered to remove the desiccant and spin-dried 2.0 g of pure product was obtained after purification by silica gel chromatography column. The yield was 83%.
Embodiment 3
[0036] Add 1.1 g BINOLOTf (2 mmol) and 0.084 g PdCl into a 20 ml reaction flask. 2 (DPPF), 0.885 g (4 mmol) of diphenylphosphonium chloride, 5 ml of anhydrous DMF, and then 0.302 g of co-catalyst iron powder. The reaction solution was heated to 110°C for 18 hours. The color analysis of the thin-layer silica gel plate shows that the raw materials have reacted completely. The reaction solution was cooled to room temperature and diluted with 20 ml of dichloromethane. The reaction solution was washed successively with 5% dilute hydrochloric acid, water, and saturated aqueous sodium bicarbonate solution, then separated, dried with anhydrous sodium sulfate, filtered to remove the desiccant and spin dried. After purification by silica gel chromatography, 0.92 g of pure product was obtained. The yield was 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com