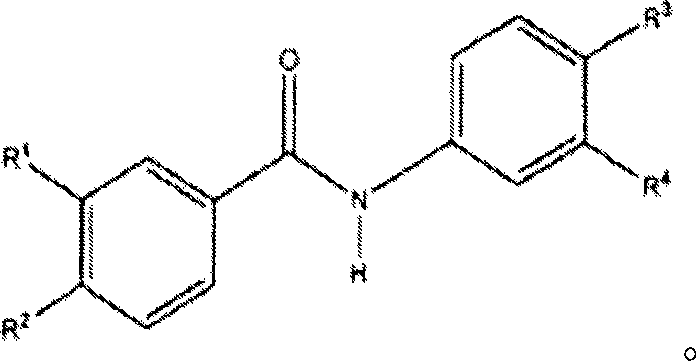
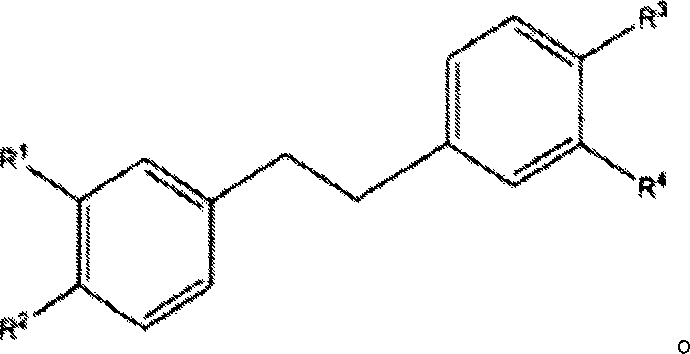
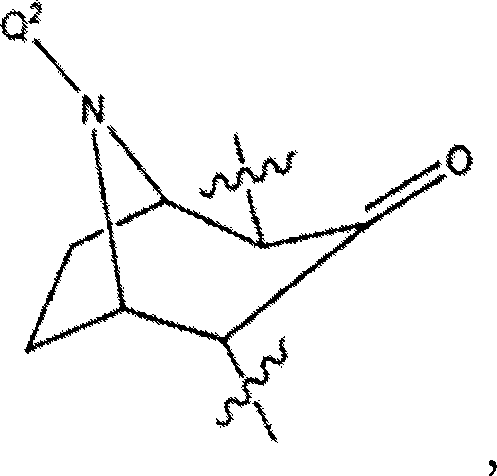
Substituted n-aryl benzamides and related compounds for treatment of amyloid diseases and synucleinopathies
A compound and substituent technology, applied in drug combination, organic chemistry, neurological diseases, etc., can solve problems such as harmfulness to patients, no obvious treatment method or effective treatment plan, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0300] Synthesis of 3-methylsulfonylamino-4-hydroxybenzoic acid 3,4-dihydroxyaniline (DC-0051-S1, also known as DC-0051-CB)
[0301]
[0302] The formation of the methanesulfonylamino derivative of amide DC-0051 can be carried out as follows: first form the well-known 3-nitro-4-methoxybenzoic acid, and then use 3,4-methylenedioxyaniline to prepare Aniline products of the preceding compounds give 3-nitro-4-methoxy-amides. This is followed by catalytic reduction via intermediate mesylation to give mesylamide, which is demethylated in a simple reaction with boron tribromide to give 3-methanesulfonylamino-4-hydroxybenzoic acid 3, 4-Dihydroxyaniline (DC-0051-S1; also known as DC-0051-CB).
[0303] A) 3-nitro-4-methoxybenzoic acid
[0304] To a suspension of p-anisic acid (3 g) in anhydrous acetic acid (20 ml) at 0°C was added concentrated nitric acid (6 ml) dropwise. The resulting clear solution was brought to room temperature and then maintained for 30 minutes. The mixture wa...
Embodiment 2
[0317] 3-Hydroxy-4-methanesulfonylamino-N-(3,4-dihydroxyphenyl)benzamide
[0318] (DC0051-S8; also known as DC-0051-DB)
[0319]
[0320] 3,4-Methylenedioxyaniline reacts with 3-methoxy-4-nitrobenzoic acid to form 3-nitro-4-methoxy-amide. Catalytic hydrogenation followed immediately by mesylation yields the mesylamino group. 3-Hydroxy-4-methanesulfonylamino-N-(3,4-dihydroxyphenyl)benzamide (DC0051-S8; also known as DC-0051) by demethylation by reaction with boron tribromide -DB).
[0321] A) 3-methoxy-4-nitro-N-(3,4-methylenedioxyphenyl)benzamide
[0322] A suspension of 3-methoxy-4-nitrobenzoic acid (0.5g) in thionyl chloride (10ml) was heated at reflux for 1 hour. The solvent was removed under vacuum to give the acid chloride as a white solid. The acid chloride was dissolved in dry dichloromethane (10ml), and pyrimidine (0.5ml) and 3,4-methylenedioxyaniline (0.4g) dissolved in dichloromethane (5ml) were added dropwise solution of the mixture. The mixture was left a...
Embodiment 3
[0333] N-(3-Methanesulfonylamino-4-hydroxyphenyl)-3,4-dihydroxybenzamide
[0334] (DC0051-S6; also known as DC-0051-AE)
[0335]
[0336]Treatment of commercially available 2-methoxy-5-nitroaniline with methylsulfonyl chloride yields the methylsulfonylamino group. Catalytic reduction of the nitro group yields the desired anilino group, while condensation with 3,4-methylenedioxybenzoyl chloride yields the aniline. Removal of the methoxy and methylenedioxy groups with boron tribromide affords N-(3-methanesulfonylamino-4-hydroxyphenyl)-3,4-dihydroxybenzamide (DC0051-S6; also known as DC0051-AE).
[0337] A) 2-Methoxy-5-nitro-methanesulfonylaminobenzene.
[0338] To a solution of 2-methoxy-5-nitroaniline (5g) in pyrimidine (25ml) at 0°C was added dropwise methanesulfonyl chloride (3.5ml) followed by pyridine (0.5ml). The mixture was maintained at 0 °C for 1 hour, then the temperature was raised to room temperature and maintained for 2 hours. The mixture was poured on ice (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com