Double chemical reflux process for exchanging nitrogen oixde and nitric acid to producing N-15
A technology of chemical exchange and nitrogen oxidation, applied in the direction of nitrogen preparation, etc., can solve the problems of low utilization rate of raw nitric acid, low value of recovered products, and impact on the cost of nitrogen-15 products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
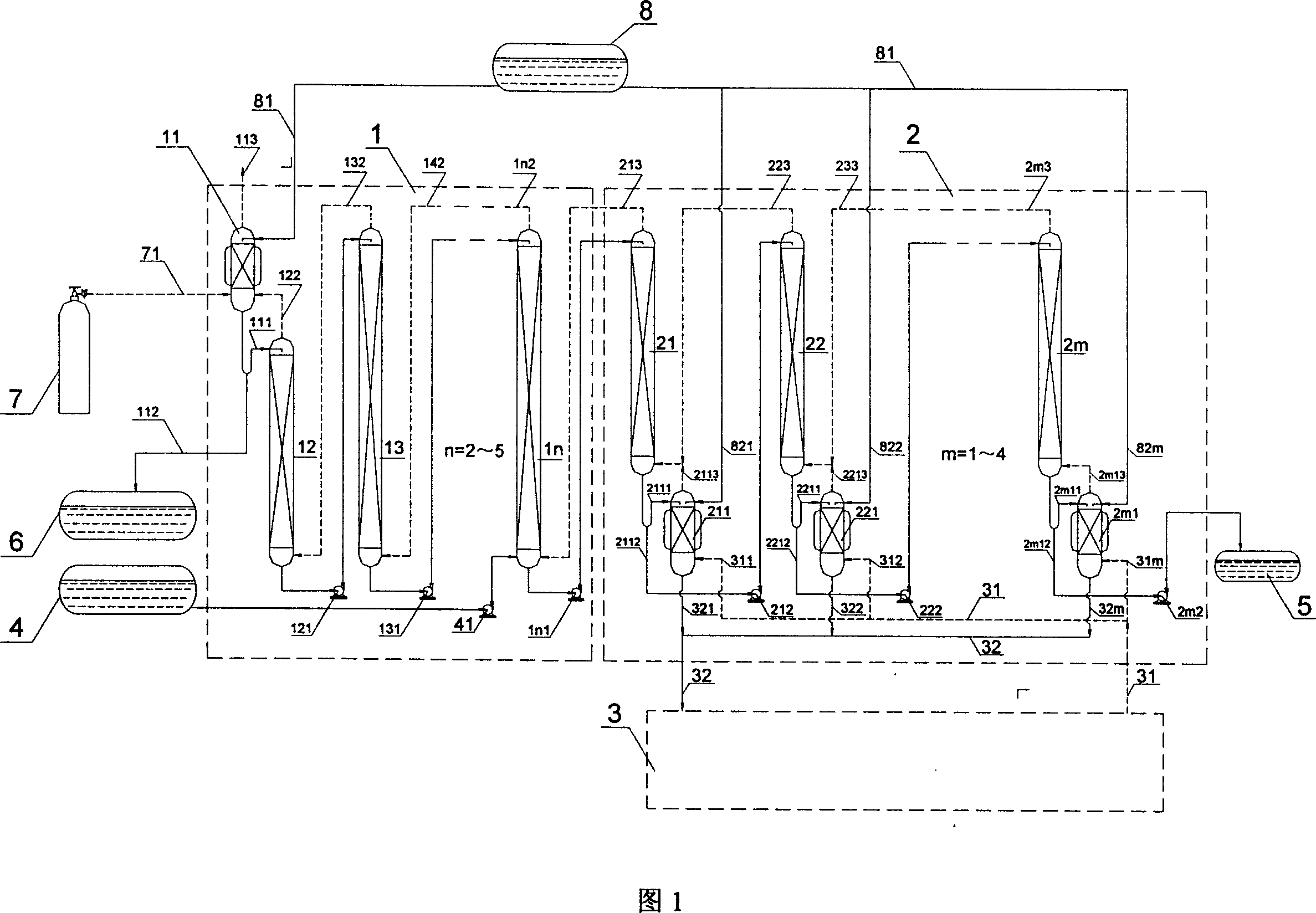
[0023] The first embodiment of the present invention, which is also a preferred embodiment of the present invention, will be described below in conjunction with FIG. 1 .
[0024] The method for producing nitrogen-15 by double chemical reflux nitric oxide / nitric acid chemical exchange described in the first embodiment of the present invention makes isotope chemical exchange of nitric oxide and nitric acid through the chemical reflux of the concentration section and the extraction section, and realizes Cycle back to the separation process.
[0025]In the concentration section 2, there are several stages of concentration towers 21, 22, ... 2m (m=1-4) connected in cascade, and corresponding bottom reflux devices 211, 221, ... 2ml and metering pump 212 , 222, ... 2m2; In the present embodiment, also utilized the sulfuric acid production device 3 in the sulfuric acid production workshop. The sulfuric acid production unit 3 introduces the sulfur dioxide into the bottom reflux device...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com