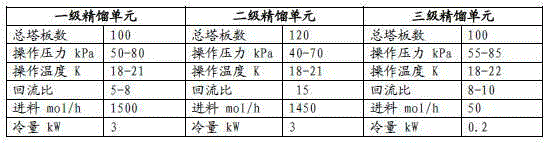
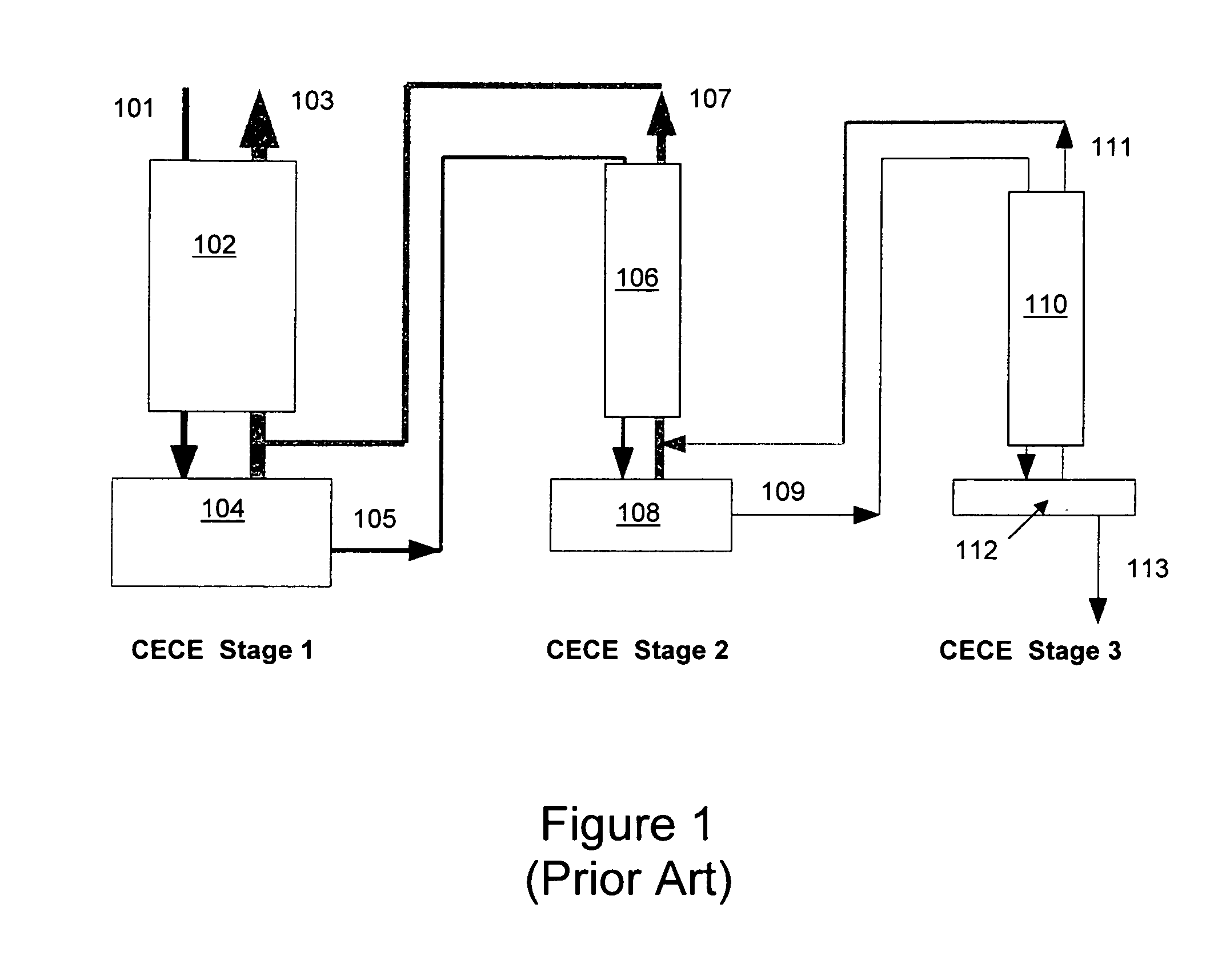
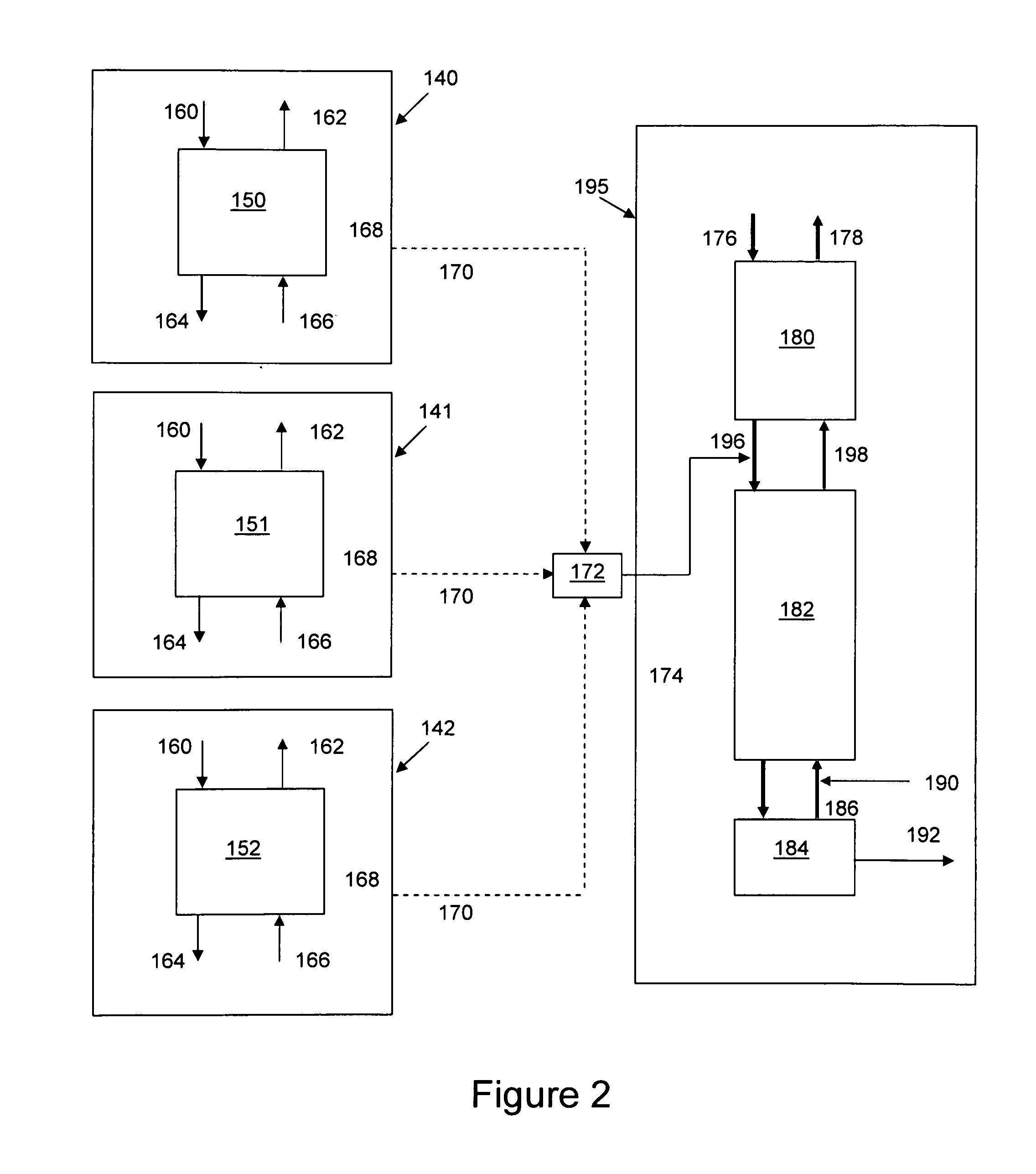
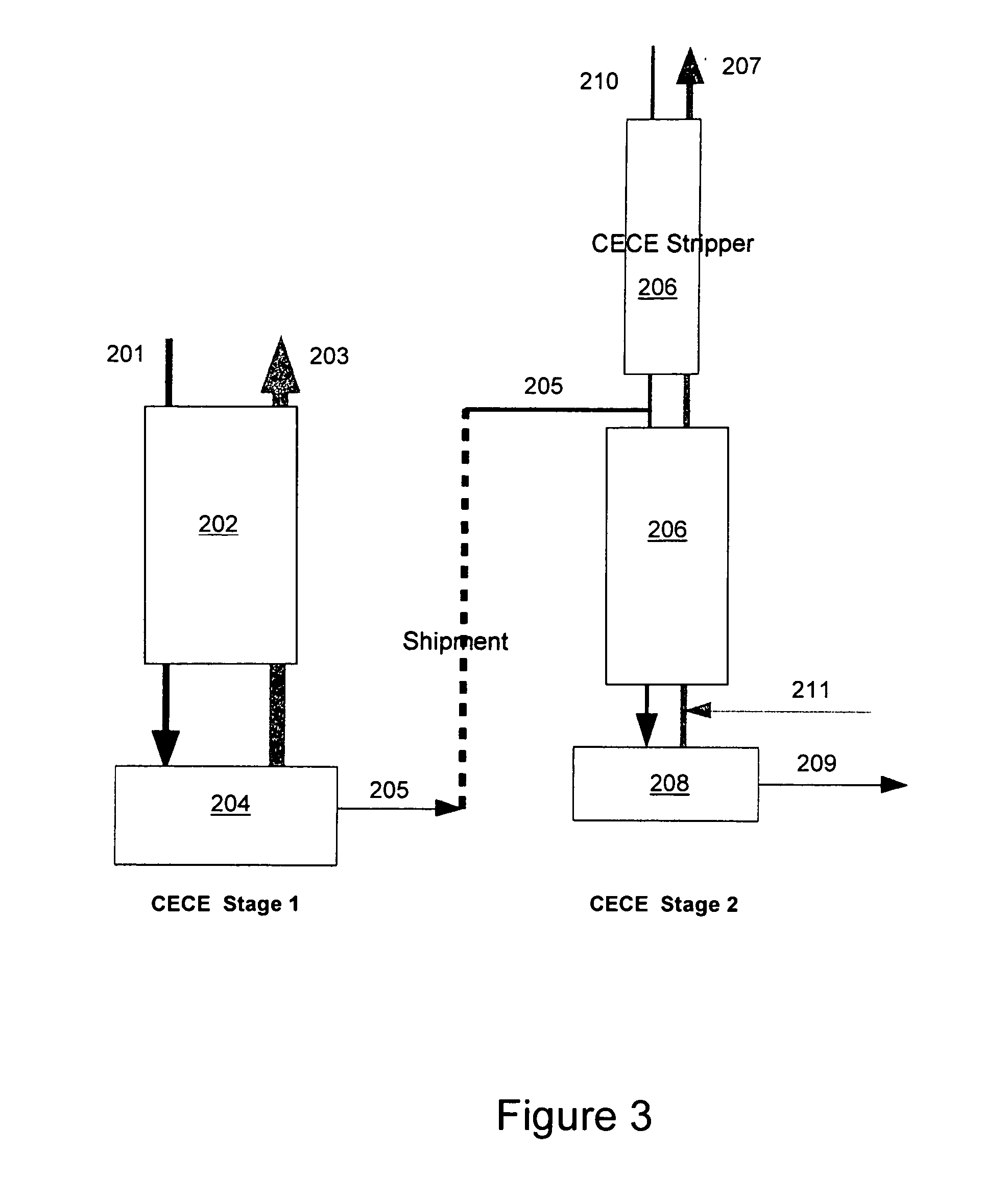
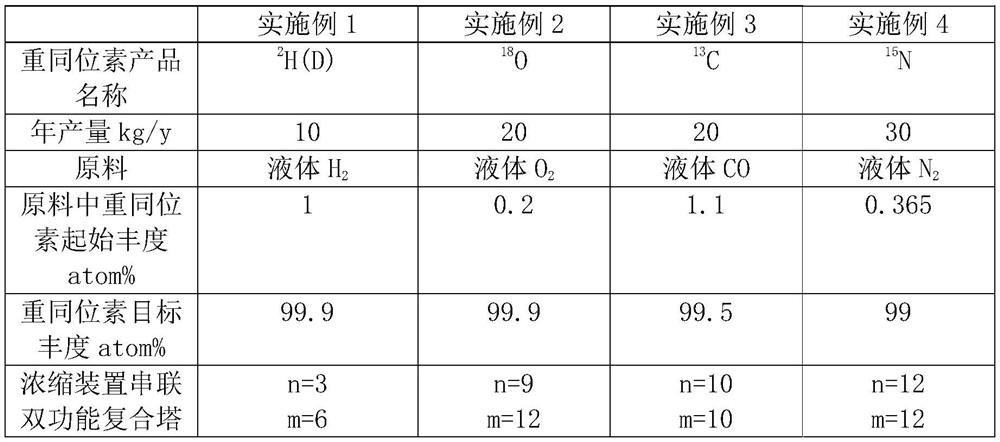
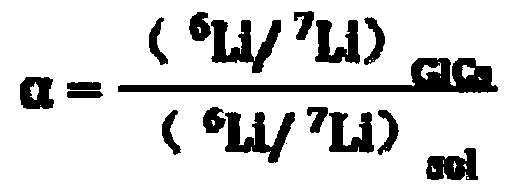Patents
Literature
44 results about "Isotope exchange" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
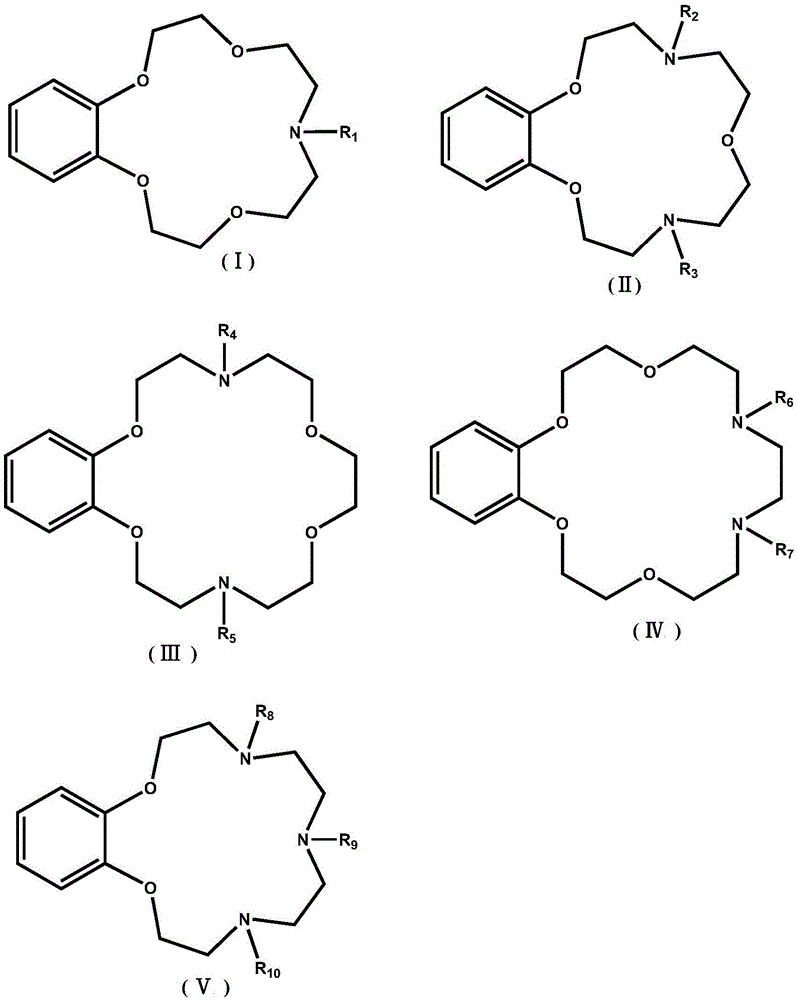
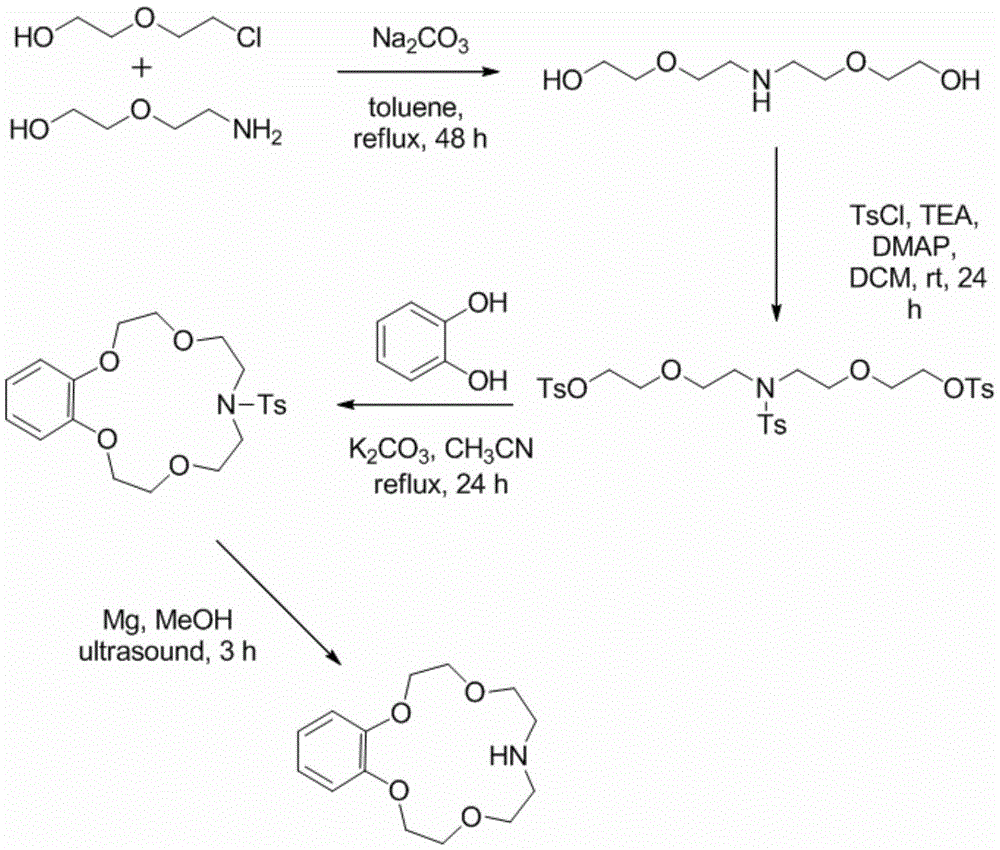
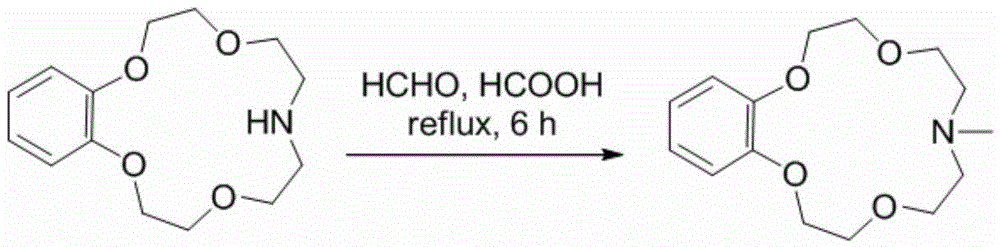
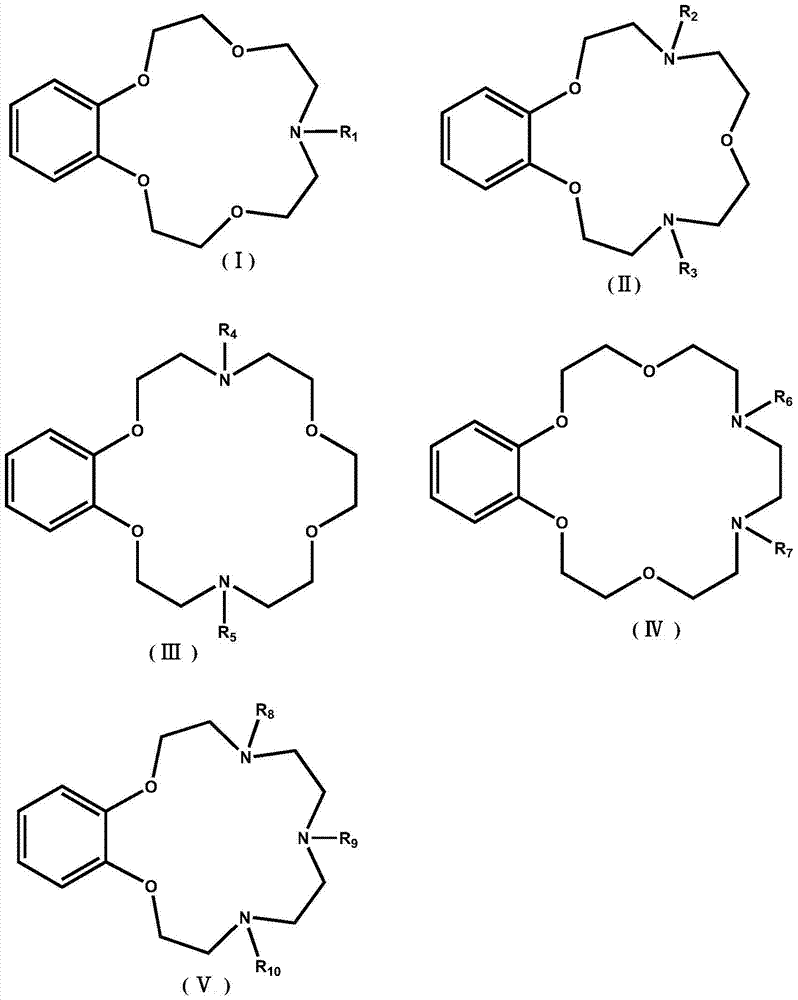
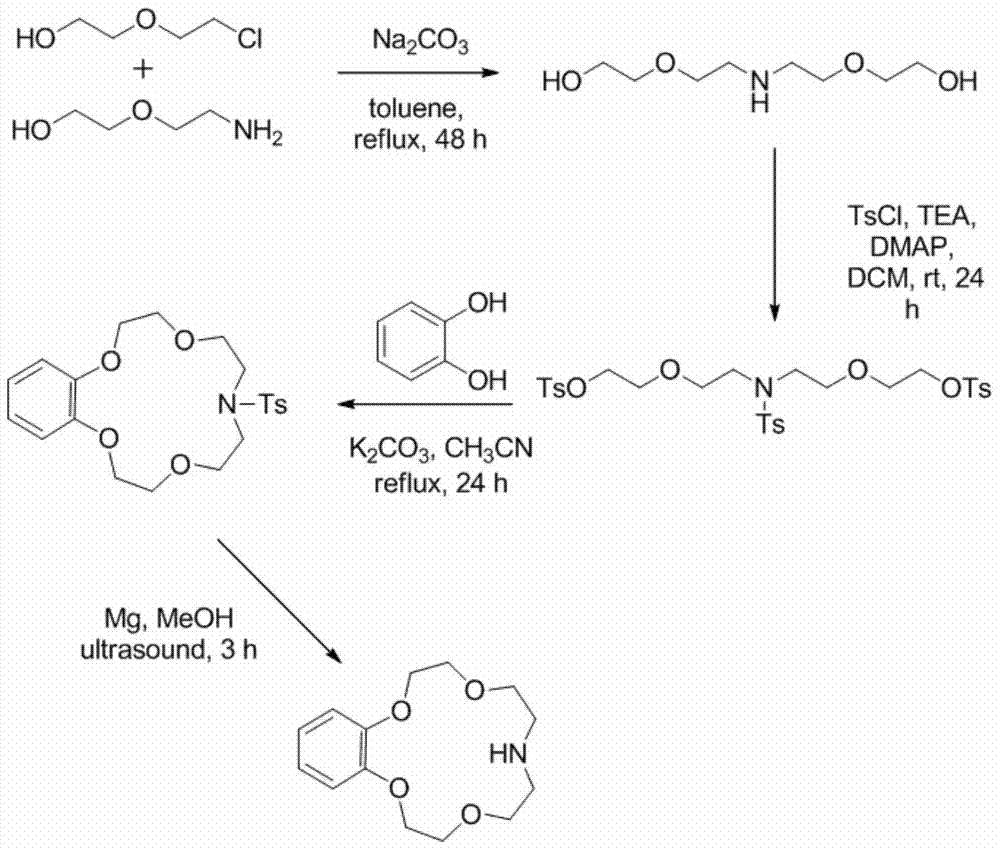
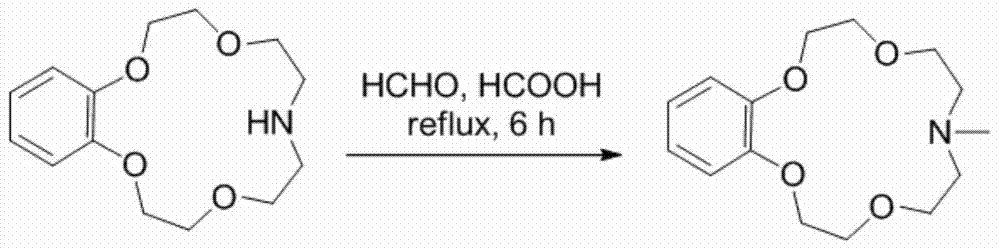
Application of benzo-azacrown ether compounds to separation of lithium isotopes
ActiveCN105561790AEfficient separationFast exchange rateOrganic chemistryIsotope separationIsotope18-Crown-6
The invention discloses application of benzo-azacrown ether compounds to separation of lithium isotopes. The benzo-azacrown ether compounds are selected from monoaza15-crown-5, bisaza15-crown-5, triaza15-crown-5 and bisaza18-crown-6. The benzo-azacrown ether compounds serving as extracting agents are dissolved in organic solvents to prepare organic phases, a lithium trifluoroacetate aqueous solution serves as an aqueous phase, and the lithium isotopes are separated at the room temperature by liquid-liquid extraction. The benzo-azacrown ether compounds are easy to dissolve in the organic solvents, efficient separation of the lithium isotopes can be realized by means of liquid-liquid extraction, and considerable separation factors, simplicity and convenience in operation, quickness in isotope exchange and technical simplicity are realized.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
Preparation method of hydrophobic catalyst for hydrogen-water isotope exchange
InactiveCN103551202AHigh strengthVarious shapes and specificationsOrganic-compounds/hydrides/coordination-complexes catalystsMicro structurePolymer science
The invention discloses a preparation method of a hydrophobic catalyst for hydrogen-water isotope exchange, which belongs to the technical field of catalyst exchange and aims at solving the problems of single shape, low utilization rate of reactive metals, and the like in an existing hydrophobic catalyst for hydrogen-water isotope exchange. The preparation method comprises the steps of suspension liquid preparation, loading, and heat treatment. The hydrophobic catalyst prepared by the method can meet the requirements of water tritium removal and heavy water purification and production on special filling configuration (in bulk, in order and the like) and performance in produced exchange columns with different scale grades. According to the method, a metal fiber felt serves as a support carrier and a foaming agent is used for modifying the micro structure of a hydrophobic membrane, the prepared hydrophobic catalyst has the characteristics of controllable shape, varied shape specifications, honeycomb-structure of the hydrophobic membrane, high utilization rate of reactive metals, high strength, and the like, and can meet the requirements of tritium removal and heavy water purification and production on filling configuration and performance in exchange columns with different scale grades.
Owner:SICHUAN INST OF MATERIALS & TECH
Preparation method and zpplication of super light water
The invention relates to the water treatment technical field, in particular to a preparing method and application of an ultra light water, wherein sulfureted hydrogen two-temperature exchange method or other alternative methods are adopted to produce ultra light water; sulfureted hydrogen two-temperature exchange method realizes separation of hydrogen isotopes through adopting multistage concatenation of cooling tower and thermal tower by use of isotope exchange reaction between sulfureted hydrogen and water; liquid raw material water enters from the top of the cooling tower and flows through the cooling tower and the thermal tower from above to below, while gas material sulfureted hydrogen is added from the part between the cooling tower and the thermal tower and circulates inside the cooling tower and the thermal tower from bottom to top inside; heavy components inside the cooling tower is transferred towards liquid phase through chemical exchange so as to be concentrated inside the liquid phase; liquid material with concentrated the heavy components completes chemical exchange with ascending gas during flowing through the thermal tower, thereby, the heavy components are transferred towards gas phase; finally, finished product with lower content of heavy hydrogen can be obtained after liquid phase material discharged from the thermal tower enters into a degassing column to removing sulfureted hydrogen. The invention has the health effects such as delaying aging, radiation resisting, resisting cell mutation, activating immunocyte, improving basal metabolism level of human body, improving sleep, enhancing physical function of male, beauty and body slimming.
Owner:丛峰松 +1
Process for production of isotopes
A process for producing an isotopically enriched compound of a desired isotope includes (a) providing a cryogenic reaction zone containing a catalyst adapted to catalyze an isotope exchange reaction at a cryogenic reaction temperature, (b) feeding to the cryogenic reaction zone an enriched mixture comprising at least a compound containing the desired isotope, wherein the enriched mixture is enriched in the desired isotope above a natural abundance of the desired isotope, (c) reacting the enriched mixture in the cryogenic reaction zone thereby forming a resulting mixture containing the isotopically enriched compound, and (d) separating the resulting mixture into an enriched product which is enriched in the isotopically enriched compound and a depleted product which is depleted in the isotopically enriched compound.
Owner:VERSUM MATERIALS US LLC
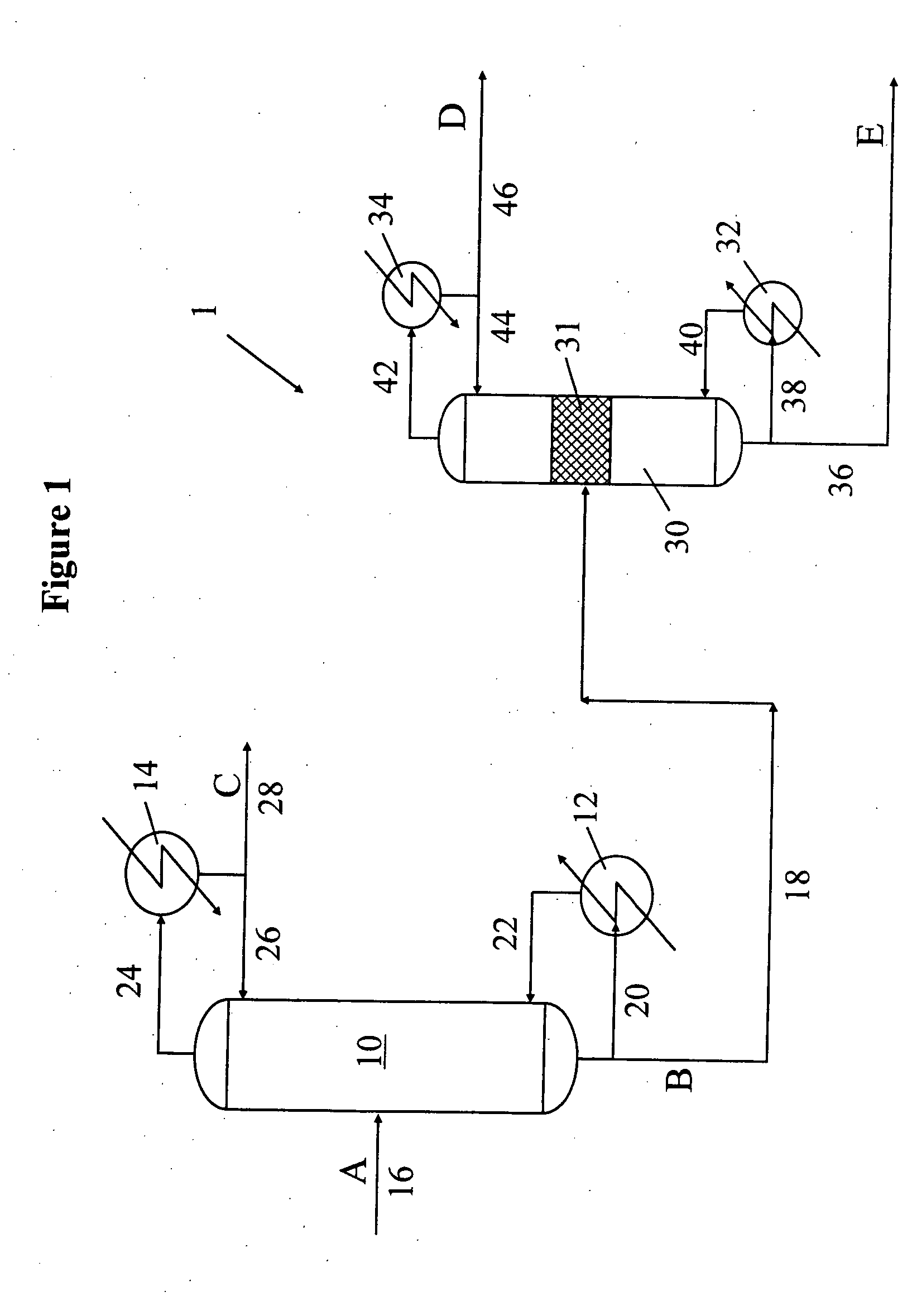
Simultaneous upgrading and tritium removal process of heavy water
ActiveCN104828778ASimple processLow one-time investment costHeavy waterPtru catalystIsotope exchange
The invention discloses a simultaneous upgrading and tritium removal process of heavy water, and belongs to the technical fields of heavy water upgrading and tritium removal. The simultaneous upgrading and tritium removal process of heavy water uses Pt / C / PTFE or Pt-SDB as a catalyst, and employs D2 to conduct isotope exchange with HDO and DTO in heavy water. The process provided by the invention avoids disadvantages of two separate devices in the traditional process for protium and tritium removal in heavy water, achieves simultaneous removal of protium and tritium, effectively reduces production cost and risks in the treatment process; in addition, by identifying key process parameters, the reaction effect of the upgrading and tritium removal process is ensured, effect of each treatment process is improved, and the economic performance of the whole process is improved.
Owner:INST OF NUCLEAR PHYSICS & CHEM CHINA ACADEMY OF
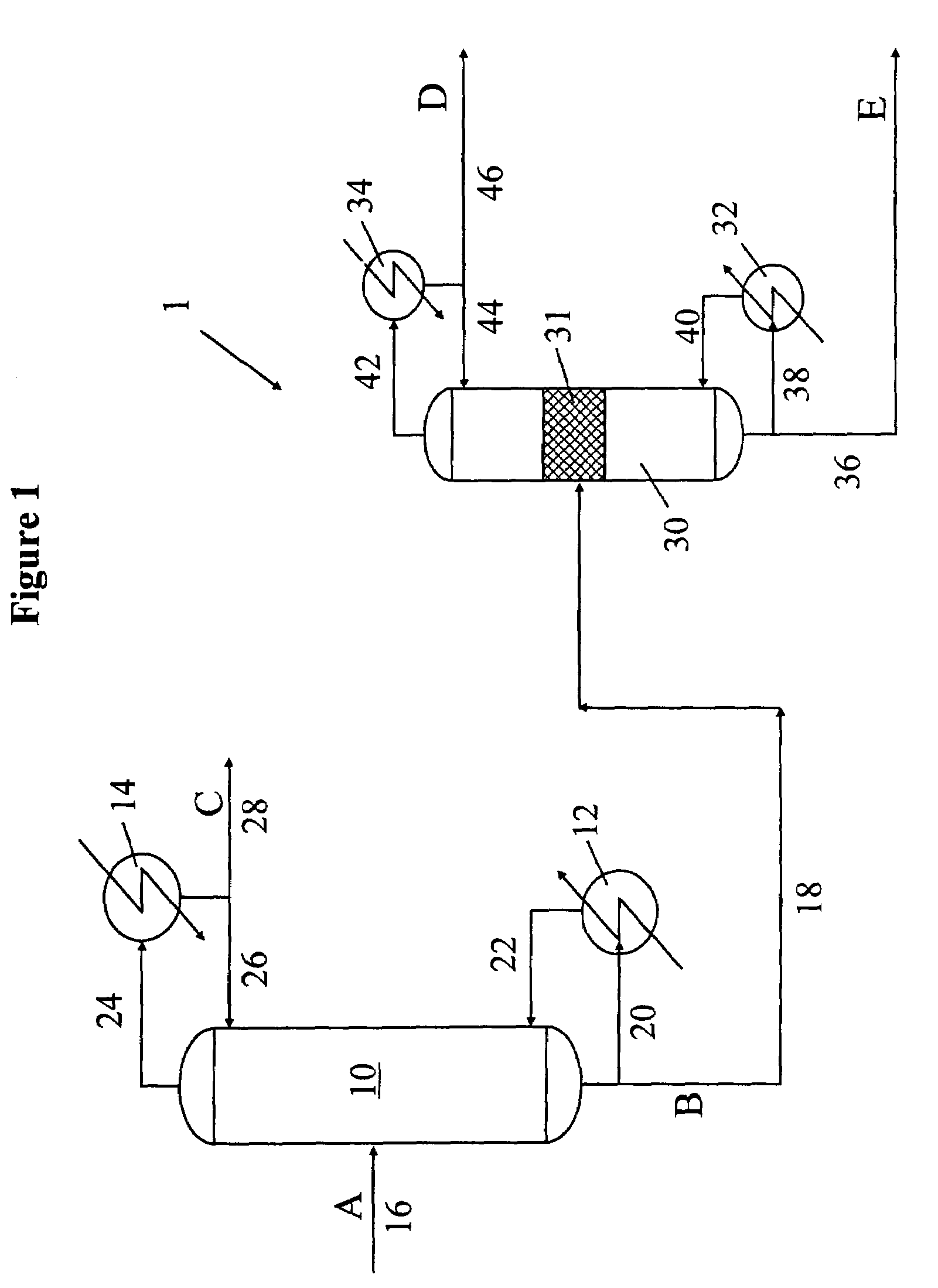
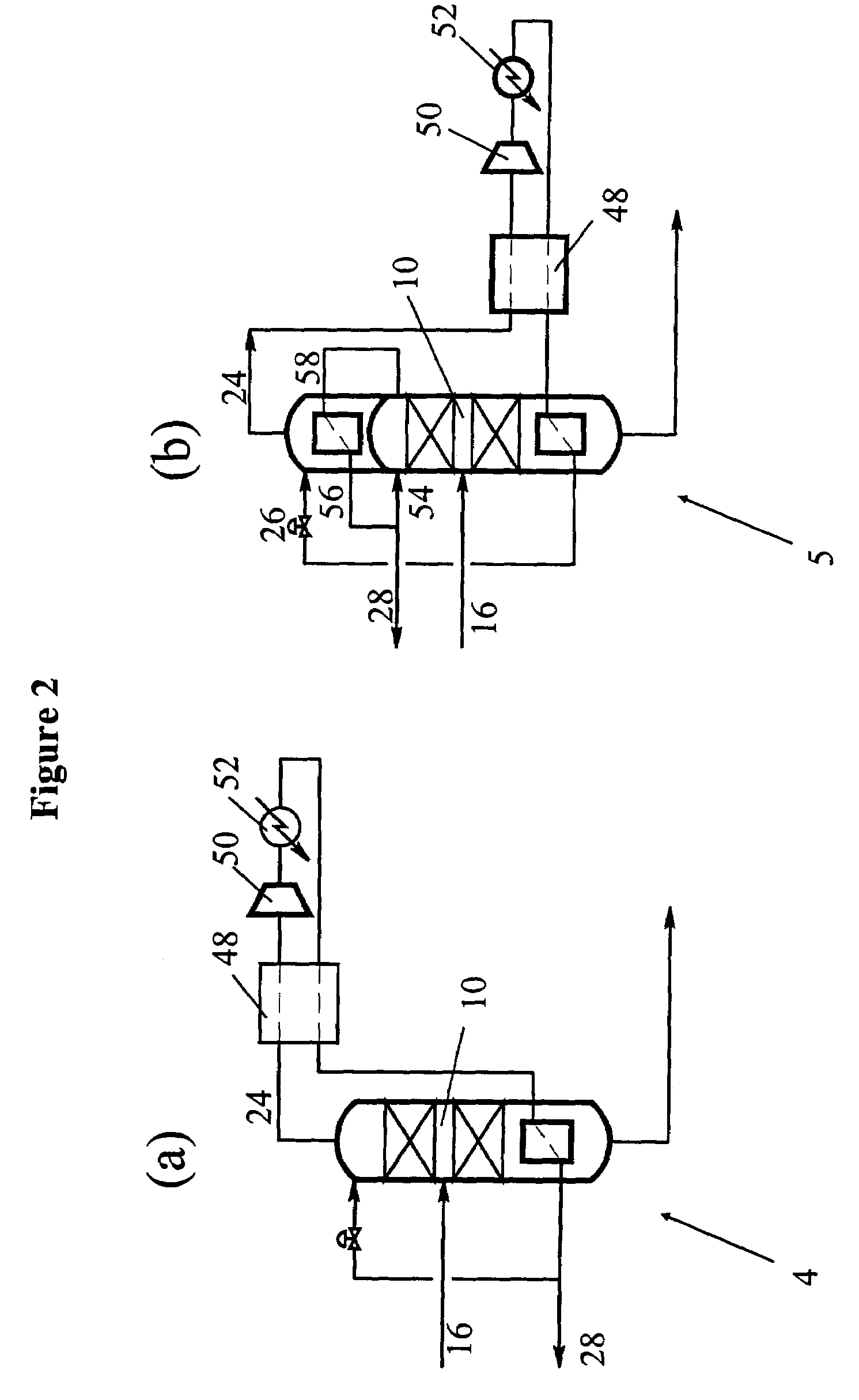
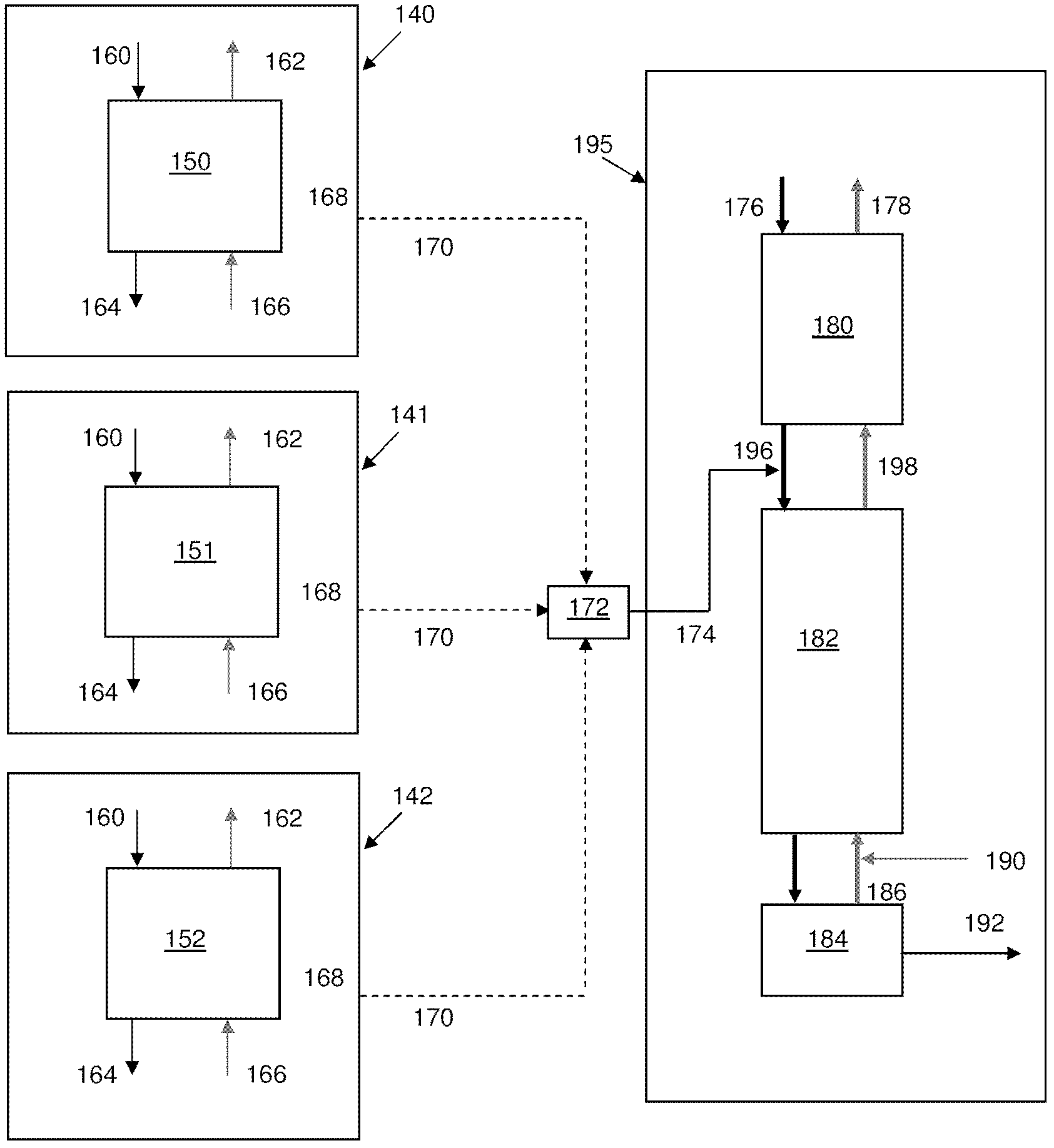
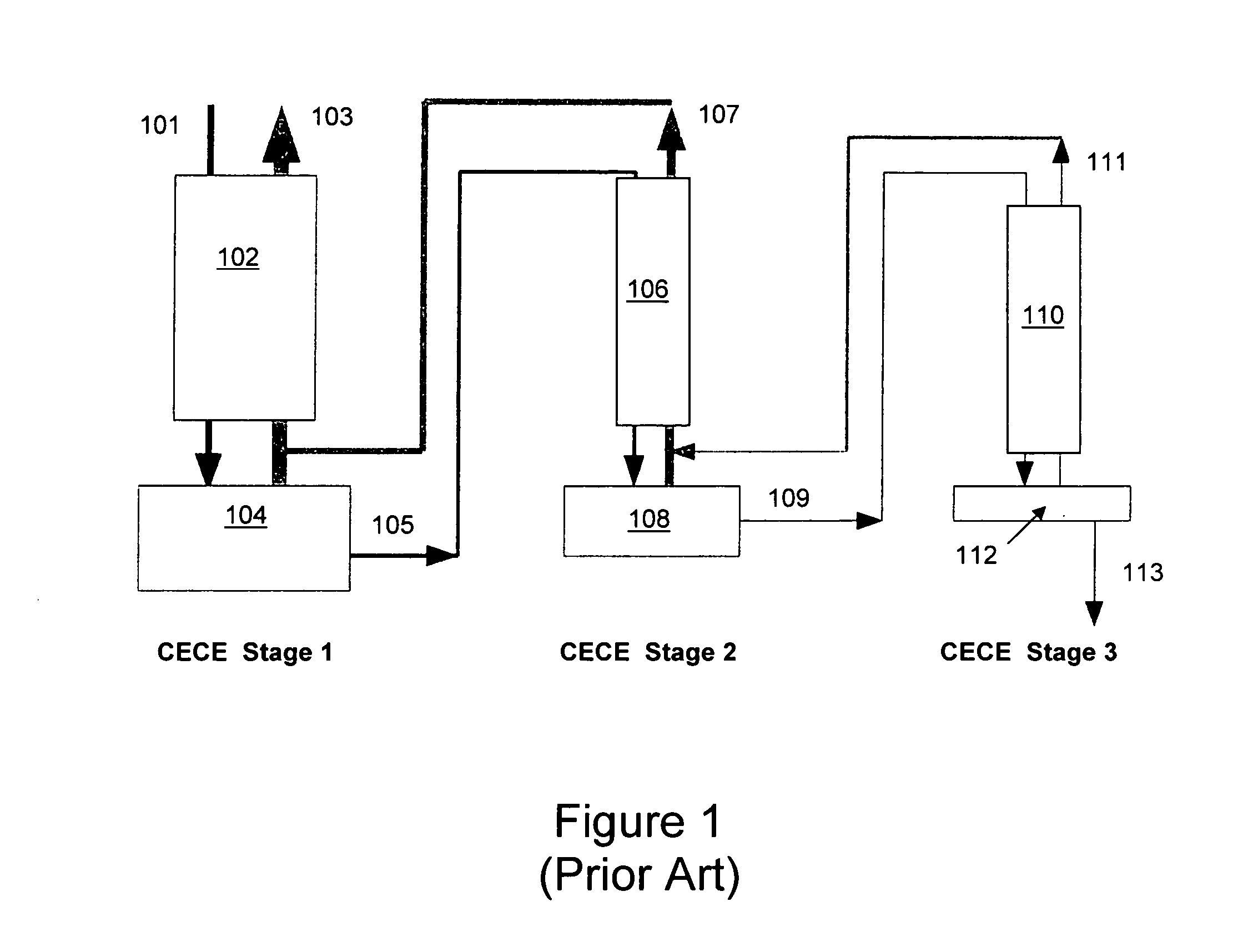
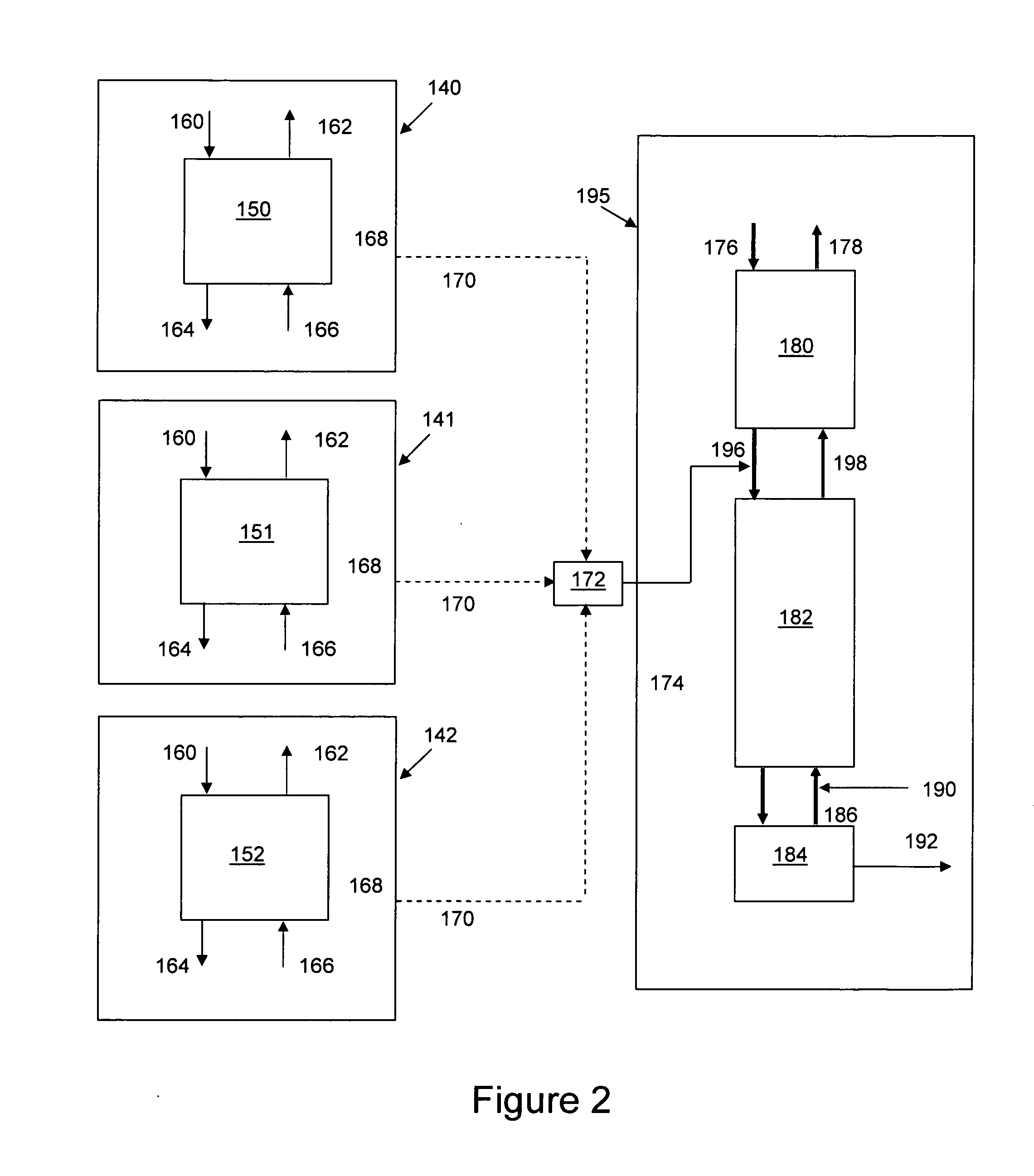
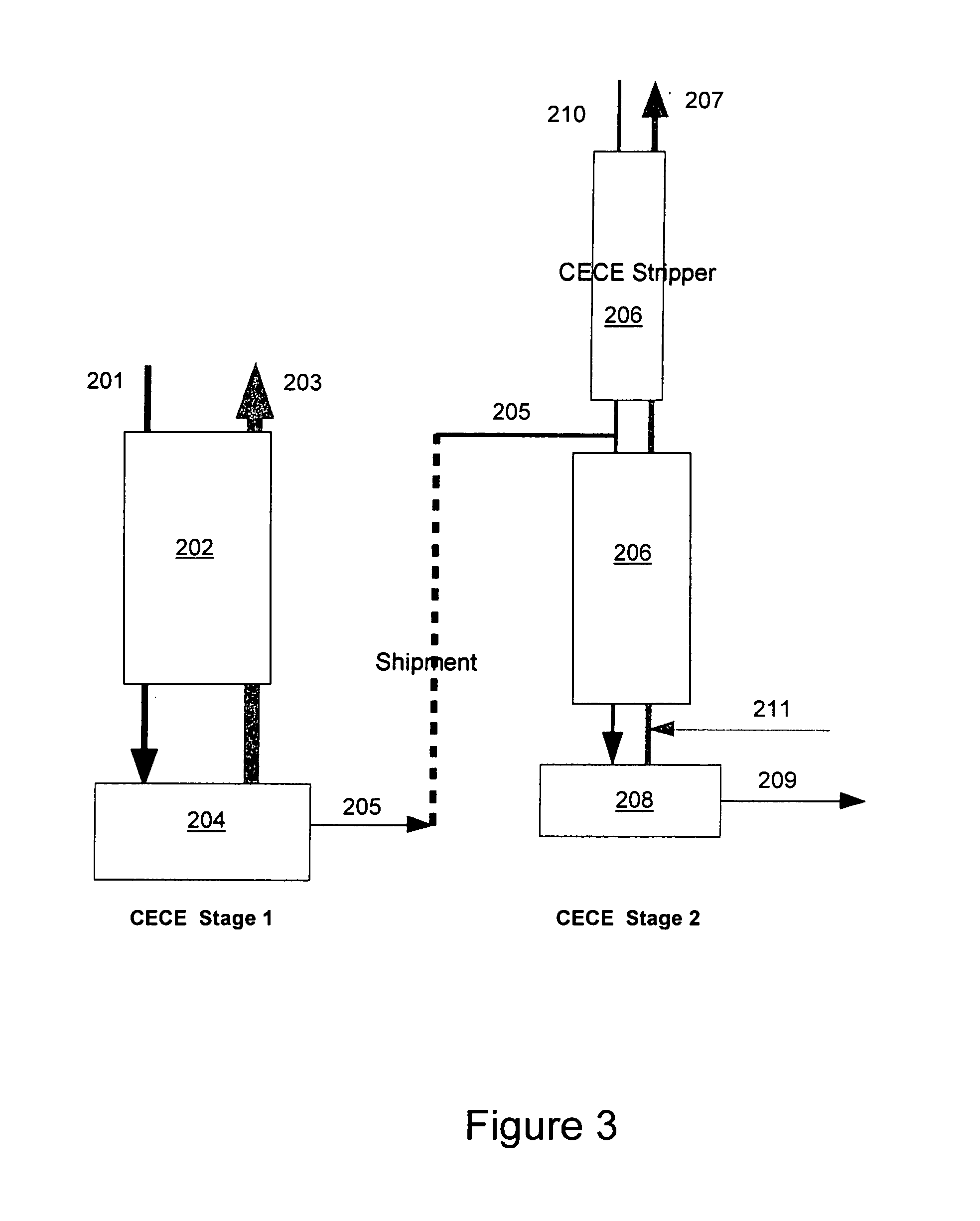
Distributed pre-enrichment method and apparatus for production of heavy water
InactiveUS20110027165A1Improve economyEasy to adaptIsotope separationEnergy based chemical/physical/physico-chemical processesPre enrichmentElectrolysis
The present invention provides a process whereby pre-enrichment of water streams using a hydrogen source and a catalytic isotope exchange method at one or more remote sites to supply water with augmented deuterium concentration to a central heavy water. This central heavy water plant could be a Combined Electrolysis and Catalytic Exchange (“CECE”) heavy water production plant or a Girdler Sulfide heavy water plant. The deuterium content of water at the remote sites is increased and provides water stream(s) with augmented deuterium concentration to feed to the central heavy water production plant. This could be a first stage of the central CECE deuterium enrichment plant, increasing its capacity for heavy water production approximately in the ratio of its enrichment above natural deuterium concentrations. By relatively simple utilization of available deuterium enrichment capacity at the remote sites, advantages are achieved from a larger scale of heavy water production at the central production plant. The invention further provides systems and methods for adapting chlorate and chlorine dioxide systems which produce hydrogen to additionally produce deuterium-enriched water.
Owner:ISOWATER CORP
Lithium isotope separation method and single-stage separation factor measurement method thereof
ActiveCN103736395AAchieve separationAvoid pollutionMaterial analysis by electric/magnetic meansIsotope separationHuman healthIsotope exchange
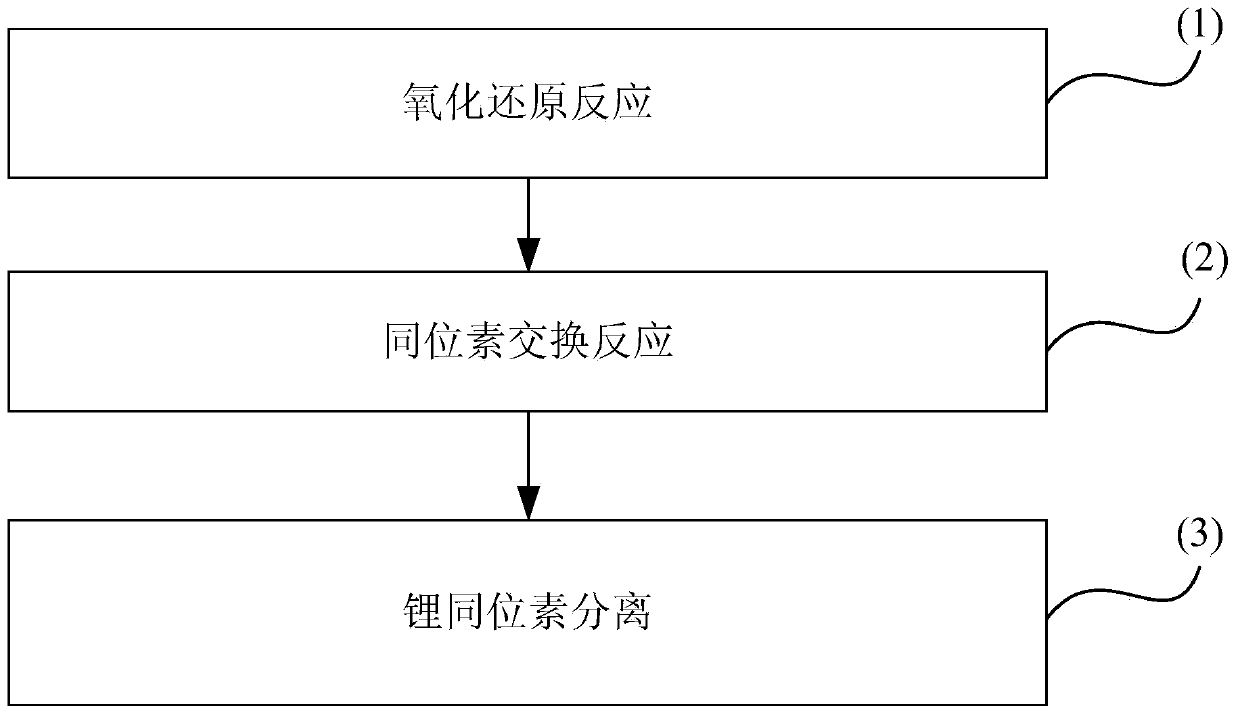
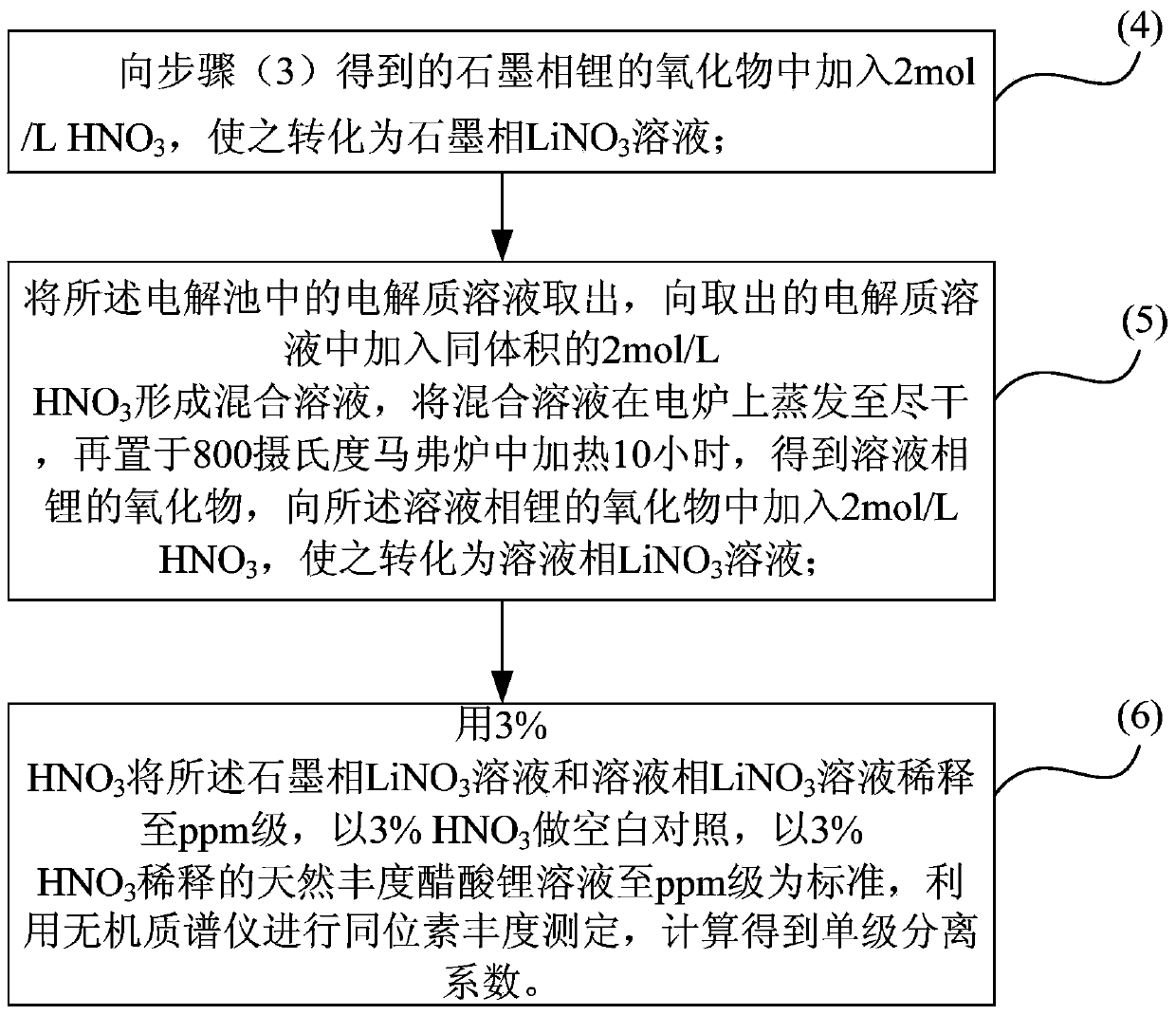
The invention belongs to the technical field of isotope separation, and in particular to a lithium isotope separation method and a single-stage separation factor measurement method thereof. According to the method, lithium isotope separation is realized by utilizing a graphite-organic electrolyte system; the method comprises three steps of oxidation-reduction reaction, isotope exchange reaction and lithium isotope separation. By the method, lithium isotope separation in a non-mercury system is realized; the pollution to an environment and the harm to human health caused by metallic mercury are avoided; the method is easy to operate; moreover, the single-stage separation factor reaches over 1.02, the separation efficiency is high, and the requirement on a single-stage separation factor of lithium isotope separation in industry can be met.
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
Process for production of isotopes
Owner:VERSUM MATERIALS US LLC
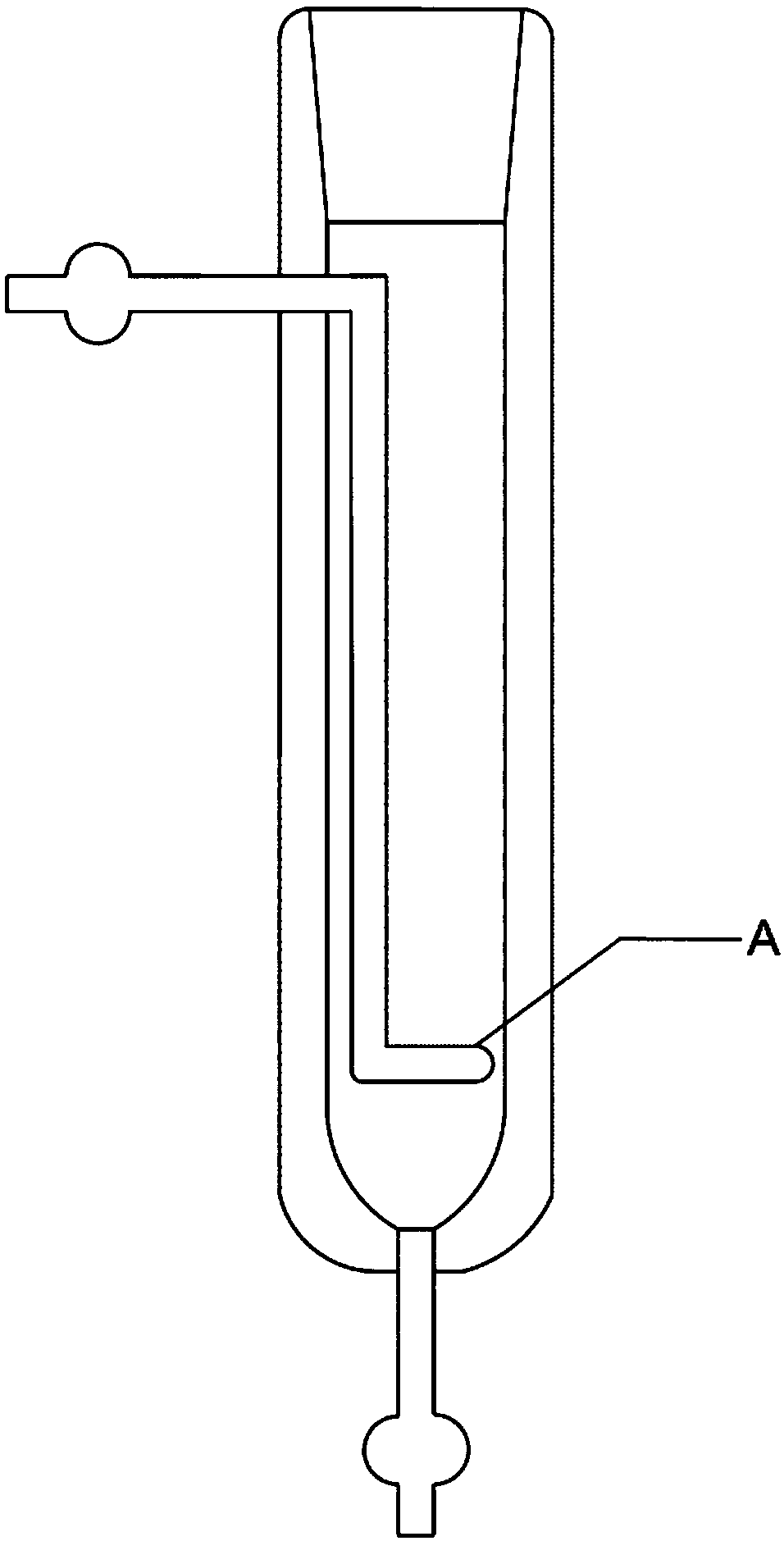
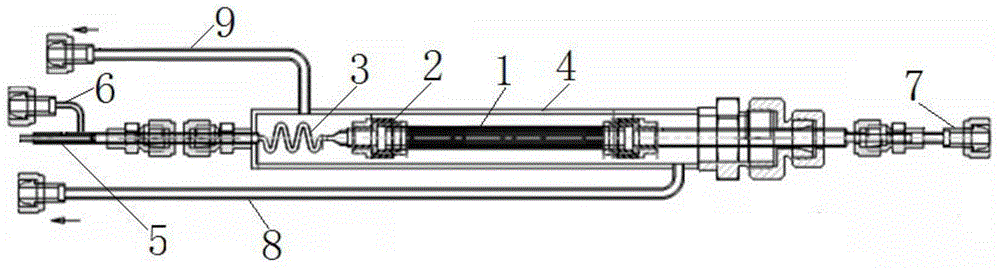
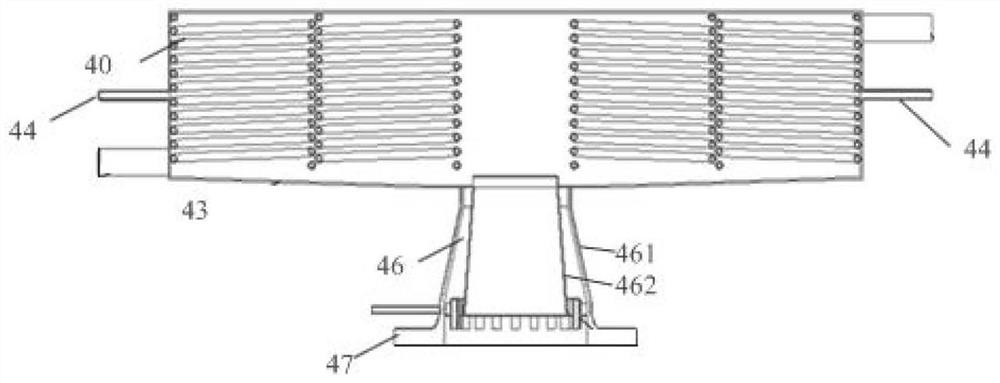
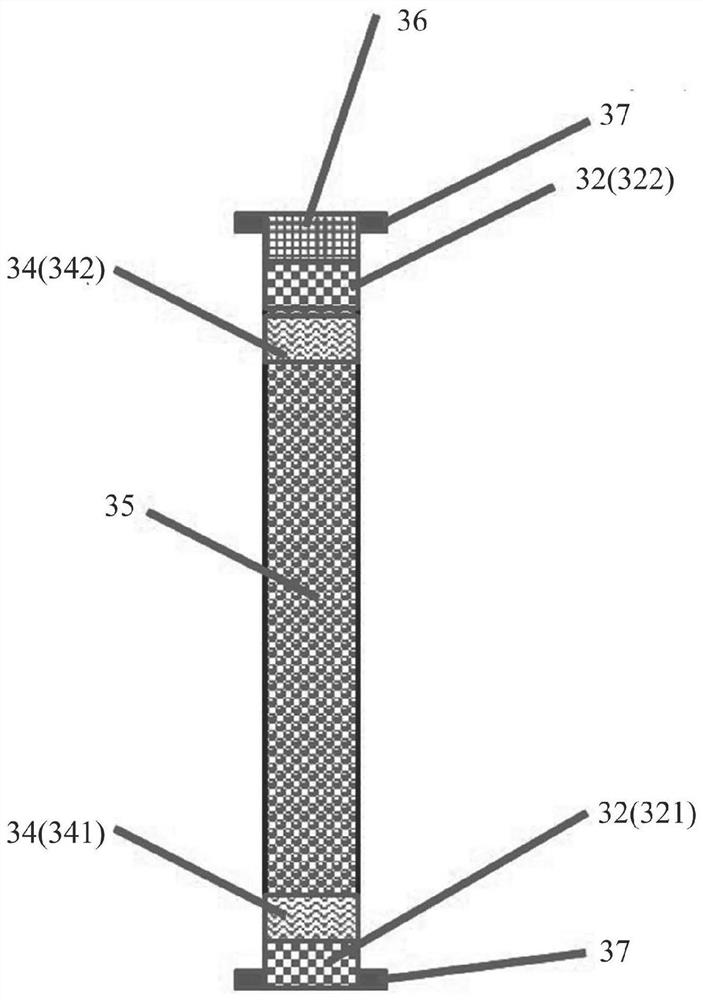
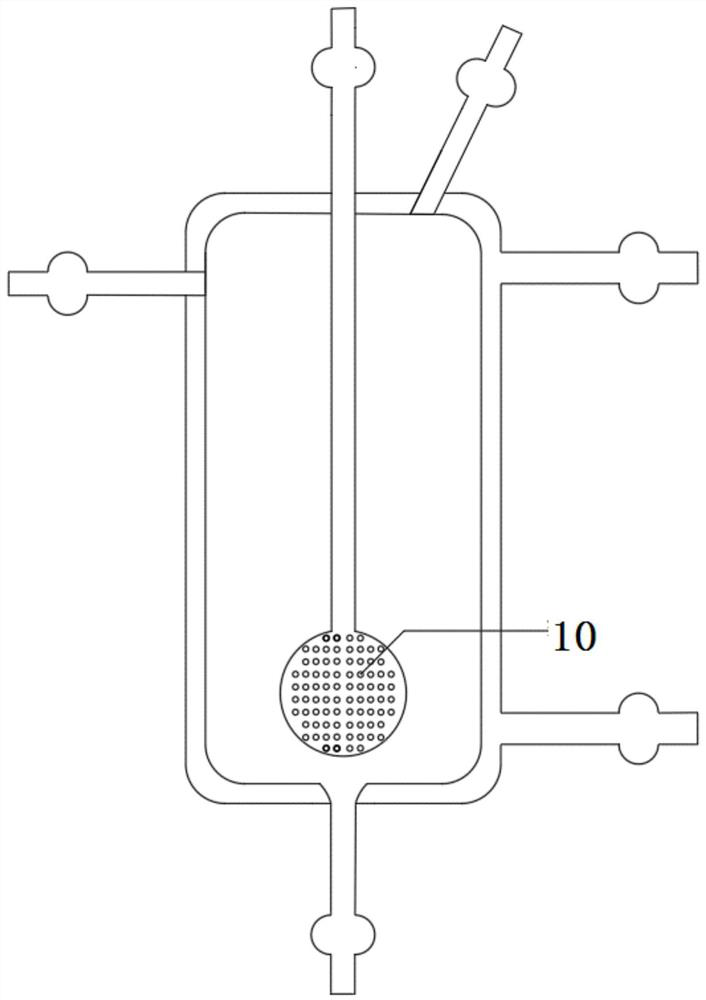
Hydrogen isotope exchange membrane reaction assembly
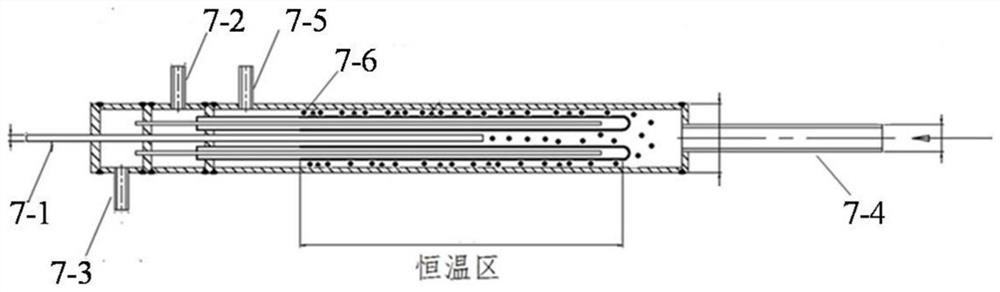
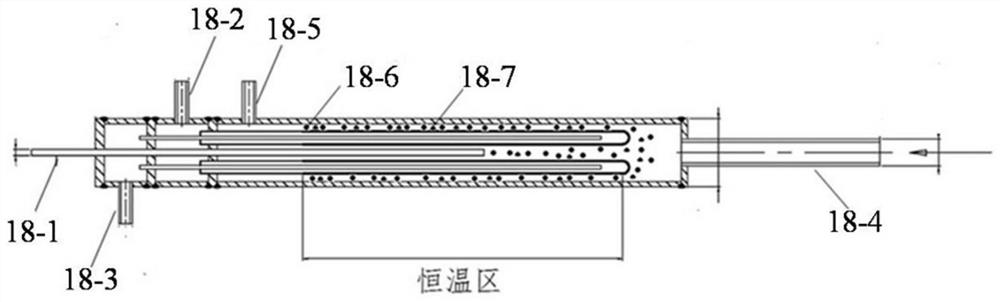
InactiveCN104129758AHigh recovery rateImprove conversion rateHydrogen isotopesHigh concentrationIsotope exchange
The invention discloses a hydrogen isotope exchange membrane reaction assembly, comprising a palladium composite membrane tube, a corrugated stainless steel tube and an external sleeve, wherein a thermocouple sleeve is in a threaded connection with one end (close to the corrugated stainless steel tube) of the external sleeve and an osmosis gas outlet is mechanically welded at the other end of the external sleeve; a branch pipe serving as a hydrogen isotope exchange raw gas inlet is arranged at the upper end of the thermocouple sleeve; a tail gas outlet is formed at the upper end of the external sleeve and close to the corrugated stainless steel tube, and a carrier gas He and Q2O raw gas inlet is formed at the lower end of the external sleeve far and is away from the corrugated stainless steel tube. According to the hydrogen isotope exchange membrane reaction assembly disclosed by the invention, a small amount of combined deuterium and tritium can be displaced and separated out by using hydrogen isotope exchange reaction, and a part of products are separated in situ out of the system during reaction, so that very high conversion rate and decontamination factors are obtained, and the recovery rate of deuterium and tritium is improved; the hydrogen isotope exchange membrane reaction assembly can be used for treating high-concentration tritium water and treating plasma ash discharge gas in fusion reaction, and can also be used for industrially producing high-purity hydrogen.
Owner:INST OF NUCLEAR PHYSICS & CHEM CHINA ACADEMY OF
Preparation method of radioactive methyl iodide
InactiveCN104591955AReduce corrosionReduce operational instabilityPreparation by halogen replacementIsotope introduction to organic compoundsSodium iodideAir cleaning
The invention belongs to the field of a nuclear air cleaning technology and relates to a novel preparation method of radioactive methyl iodide for detecting active carbon performances and iodine absorber efficiency of a nuclear air cleaning system. The preparation method utilizes an alcohol-water mixed solution of nonradioactive iodomethane as a reagent and comprises the following steps of mixing a prepared nonradioactive iodomethane reactive liquid and a radioactive sodium iodide solution to obtain a radioactive methyl iodide generation liquid, standing the generation liquid for some time so that iodine isotopic exchange is finished and radioactive iodomethane is obtained, putting the radioactive iodomethane generation liquid into a methyl iodide generator, carrying out radioactive iodomethane generation, and injecting the product into a test loop for detecting iodine absorber efficiency. The preparation method prevents a risk caused by use and operation of a large amount of dimethyl sulphate as a highly toxic product, reduces radioactive methyl iodide generator pipe corrosion caused by a test reagent and instability of generator operation caused by corrosion, reduces maintenance frequency of the methyl iodide generator and prolongs a service life of the methyl iodide generator.
Owner:CHINA INST FOR RADIATION PROTECTION
Method for manufacturing catalyzer
InactiveCN101108341AImprove performanceSame exchange effectCatalyst carriersMetal/metal-oxides/metal-hydroxide catalystsPolyvinyl alcoholPolymerization
The invention relates to a production method of a hybrogen to water (liquid) hybrogen isotope exchange catalyst, which is characterized in that: firstly, make polyvinyl alcohol aqueous solution as compound aqueous phase and toluol and other organic solvent as pore agent, under the catalyzing of ABIN initiator to realize mass polymerization of divinylbenzene to produce polymer porous resin ball, then add the polymer porous resin ball into chloroplatinic acid ethanol solution to soak according to certain proportion, after drying, restoring and cooling to produce lyophobic catalyst. The invention is characterized in that: the technics has good repeatability, high catalyst activity, strong anti-Beta irradiance, and wide application range, which is not only suitable for extracting pure tritium in light water containing tritium of light water reactor, but also suitable for extracting pure tritium in heavy water containing tritium of heavy water reactor, also used for extracting pure tritium in liquid radioactivity outflow (nuclear waste) of nuclear power reactor, and can be used to produce heavy water by the way of hybrogen to water (liquid) dual temperature exchanging method.
Owner:柯香文
Distributed pre-enrichment method and system for production of heavy water
The present invention provides a process whereby pre-enrichment of water streams using a hydrogen source and a catalytic isotope exchange method at one or more remote sites to supply water with augmented deuterium concentration to a central heavy water. This central heavy water plant could utilize any suitable heavy water production technology, including the Combined Electrolysis and Catalytic Exchange ("CECE") heavy water production plant and Girdler Sulfide process. The deuterium content of water at the remote sites is increased and provides water stream(s) with augmented deuterium concentration to feed to the central heavy water production plant. This could be a first stage of the central CECE deuterium enrichment plant, increasing its capacity for heavy water production approximately in the ratio of its enrichment above natural deuterium concentrations. The invention further provides systems and methods for adapting chlorate and chlorine dioxide systems which produce hydrogen to additionally produce deuterium-enriched water.
Owner:ISOWATER CORP
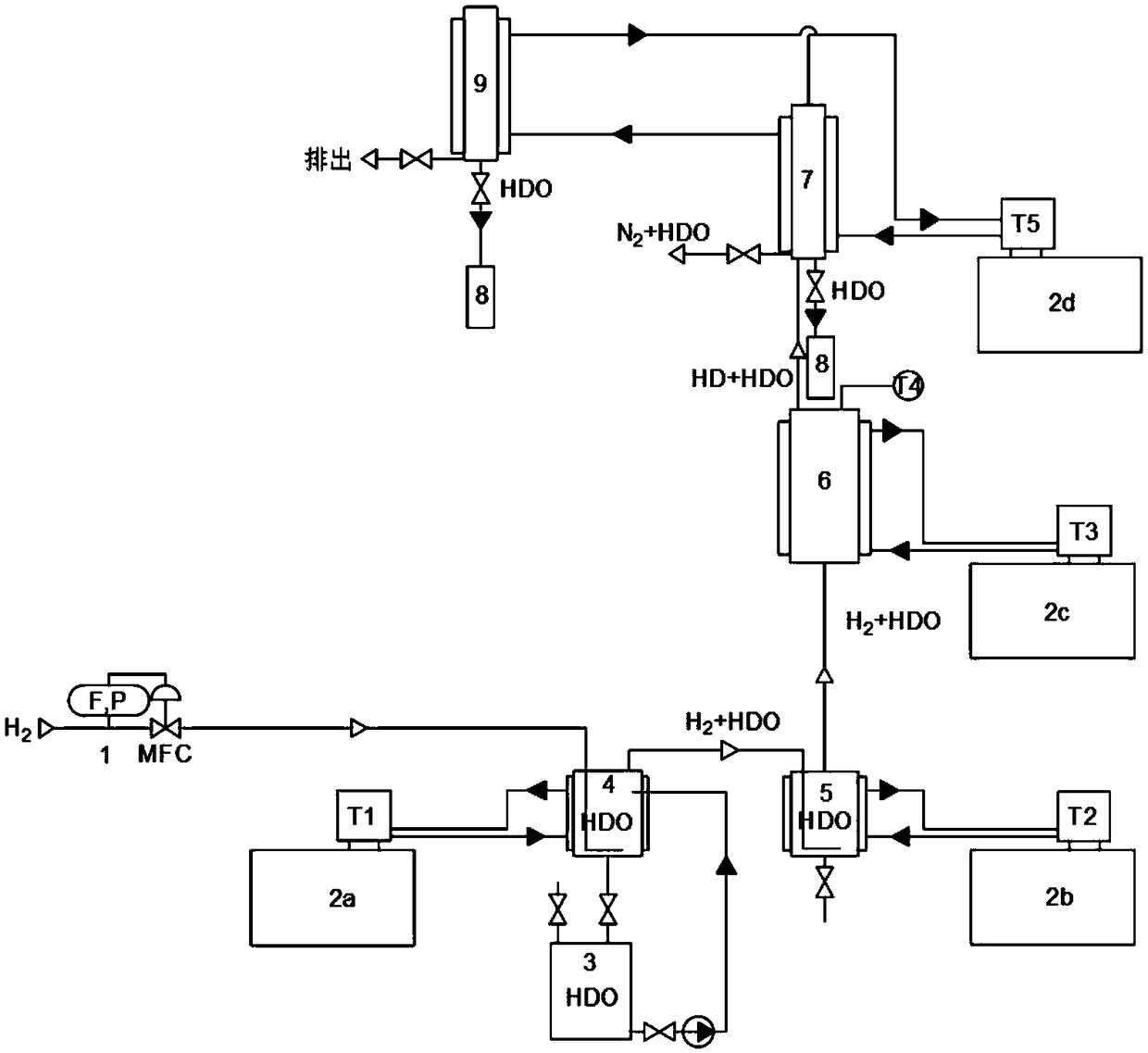
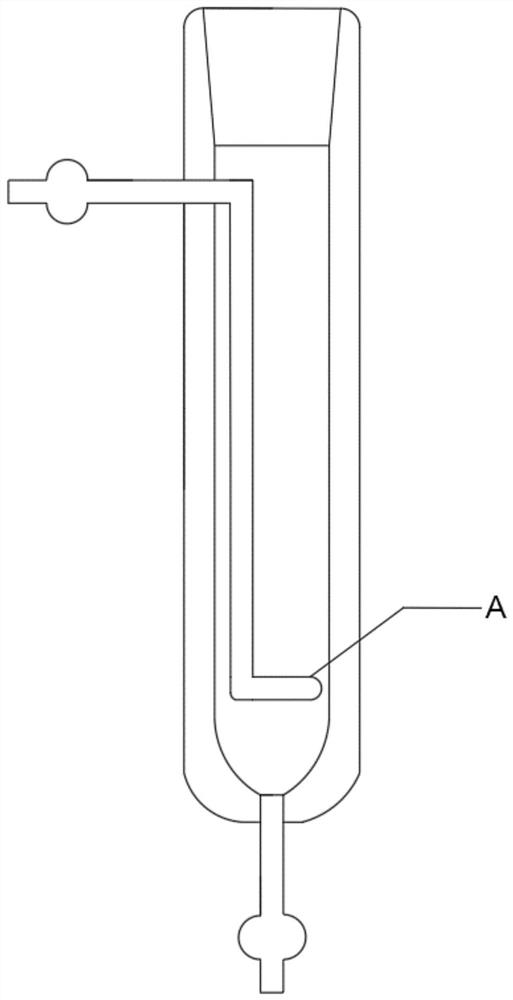
Device and method for evaluating activity of hydrophobic catalyst through hydrogen isotope gas phase exchange
ActiveCN108802267ATargetedEasy to operateChemical analysis using catalysisLiquid nitrogen coolingGas phase
The invention provides a device and a method for evaluating activity of a hydrophobic catalyst through hydrogen isotope gas phase exchange. The device comprises a gas control unit, a water saturationunit, a catalytic reaction system, a sample collection unit and a temperature control system, wherein the water saturation unit comprises a primary water saturator and a secondary water saturator; thecatalytic reaction system comprises a reaction pipe; the sample collection unit comprises a condensation pipe for carrying out condensed collection on water samples containing deuterium and a cold trap for carrying out liquid nitrogen cooling and collection on the water samples containing the deuterium; the temperature control system consists of four independent temperature control units which are respectively used for controlling temperatures of the primary water saturator, the secondary water saturator, the reaction pipe and the condensation pipe. According to the device and the method disclosed by the invention, the hydrogen gas passes through the saturated deuterium-containing water, and performs gaseous hydrogen isotope exchange reaction on the catalyst bed, the deuterium-containingwater is collected by adopting backflow condensation and / or cold trapping, so that the content of the deuterium in the water before exchange is quantitatively analyzed through an infrared method, andthe catalytic performance of the catalyst is evaluated. The device and the method have the advantages that an operation is simple, and the device and the method are suitable for evaluating the performance of the catalyst in the experiment and research stage.
Owner:HARBIN ENG UNIV
Double chemical reflux process for exchanging nitrogen oixde and nitric acid to producing N-15
InactiveCN1986391AIncrease profitReduce dosageNitrogen preparationHigh concentrationIsotope exchange
The double chemical reflux process for exchanging nitrogen oxide and nitric acid to produce N-15 belongs to the field of high concentration N-15 nitric acid producing technology. Through the chemical reflux in the concentration section and the extraction section, nitrogen oxide and nitric acid produce chemical isotope exchange and circular reflux process is realized. In the extraction section, tail gas nitrogen oxide is recovered with deionized water and oxygen as material in the top refluxer to obtain N-14 nitric acid, and most of the recovered nitric acid is refluxed to the extracting tower for nitrogen isotope exchange to restore the N-15 isotope concentration to natural abundance. The recovered nitric acid is then merged with material nitric cid before being used as the material for the concentration section.
Owner:杨国华
System and Method for Expression Proteomics Based on Isotope Ratio Modification
InactiveUS20080038194A1In-vivo radioactive preparationsOrganic chemistryElemental compositionIsotope exchange
The present invention relates to modification of isotope ratios in peptides and polypeptides as an alternative to full isotopic exchange for coding in proteomics. Subtle modification of isotope ratio does not compromise protein identification experiments and can thus provide elemental composition data for accurate isotope ratio decoding. Application of subtle modification of isotope ratio proteomics (SMIRP) offers a convenient approach to in vivo isotope coding.
Owner:CEDARS SINAI MEDICAL CENT
A hydrogen isotope exchange membrane reaction module
InactiveCN104129758BHigh recovery rateImprove conversion rateHydrogen isotopesHigh concentrationIsotope exchange
Owner:INST OF NUCLEAR PHYSICS & CHEM CHINA ACADEMY OF
Application of Benzoazepine Crown Ether Compounds in Separation of Lithium Isotopes
ActiveCN105561790BEfficient separationFast exchange rateOrganic chemistryIsotope separationSeparation factorRadiochemistry
The invention discloses application of benzo-azacrown ether compounds to separation of lithium isotopes. The benzo-azacrown ether compounds are selected from monoaza15-crown-5, bisaza15-crown-5, triaza15-crown-5 and bisaza18-crown-6. The benzo-azacrown ether compounds serving as extracting agents are dissolved in organic solvents to prepare organic phases, a lithium trifluoroacetate aqueous solution serves as an aqueous phase, and the lithium isotopes are separated at the room temperature by liquid-liquid extraction. The benzo-azacrown ether compounds are easy to dissolve in the organic solvents, efficient separation of the lithium isotopes can be realized by means of liquid-liquid extraction, and considerable separation factors, simplicity and convenience in operation, quickness in isotope exchange and technical simplicity are realized.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
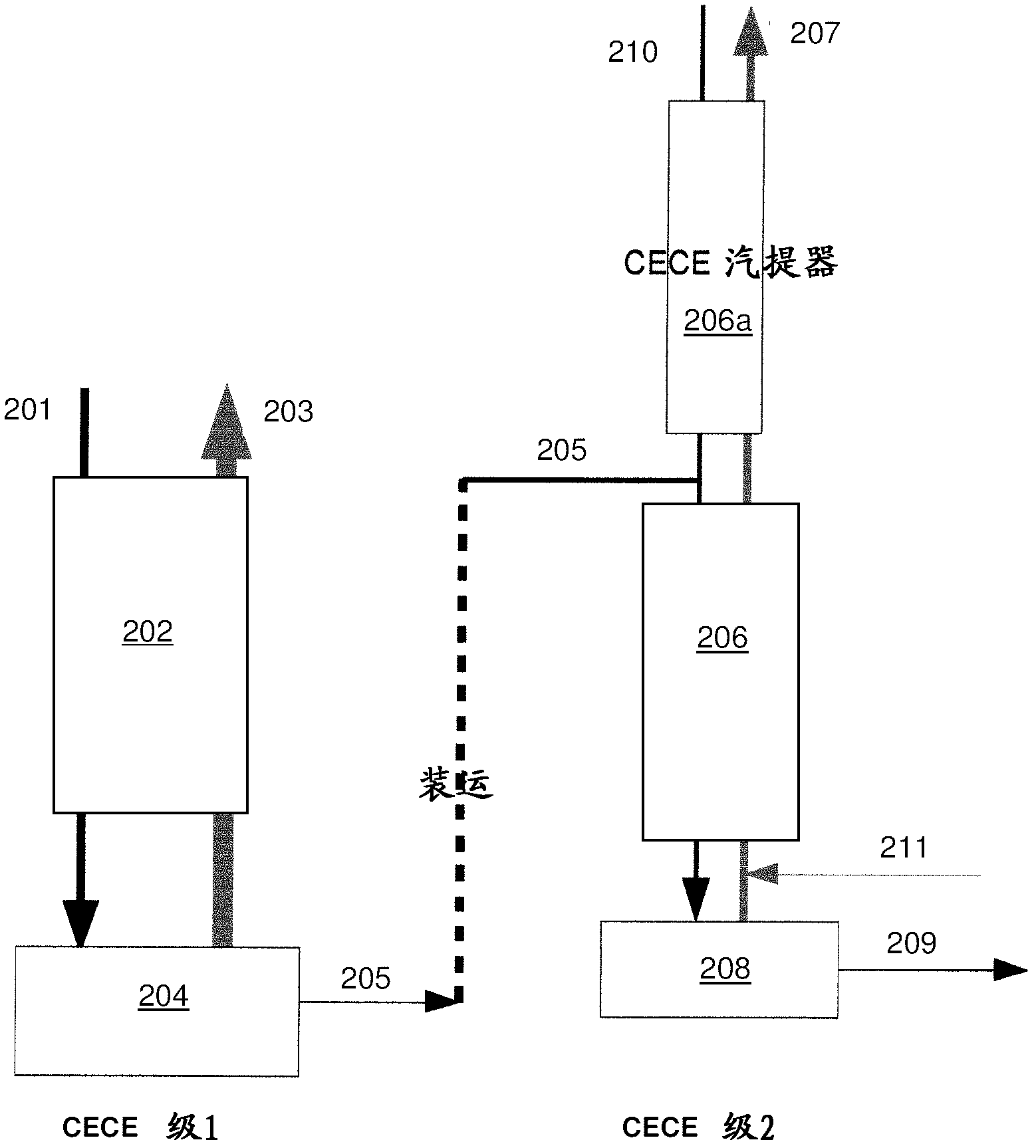
Method and system for producing high concentration carbon dioxide of carbon-13
InactiveCN101054177AReduce manufacturing costAvoid clogging accidentsCarbon compoundsAir quality improvementHigh concentrationSolvent
The present invention relates to a method and a system for preparing C-13 carbon dioxide with a high concentration, belonging to the preparing technical field of stable isotope C-13. The present invention discloses a method and system for the industrialized production of high concentration C-13; wherein the concentration of isotope is more than 99%, the natural carbon dioxide serves as raw material, the carbon dioxide / dinbutylamine / octane serves as the isotope exchange system, and a cold-hot refluxing method is adopted. In the present invention, the technical measures of raw gas presaturation, extractor segment and kettle liquid circulation are adopted, thereby the dosage of raw gas and the solvent consumption are reduced by 99% or so, and the concentration of the working solution of the system is stabilized, so that the concentration effect of the isotope is improved and the production cost is reduced. Furthermore, a hot reflux tower with a direct heat exchange-cool liquid leaching segment is adopted, and the operating temperature and the control requirement are reduced, thereby the crystallizing choking phenomenon is prevented, the operating is convenient and stable, and the energy consumption is saved.
Owner:杨国华
Chlorate and chlorine dioxide systems adapted for the production of deuterium enriched water
InactiveUS20110027166A1Improve economyEasy to adaptCellsIsotope separationPre enrichmentChlorine dioxide
The present invention provides a process whereby pre-enrichment of water streams using a hydrogen source and a catalytic isotope exchange method at one or more remote sites to supply water with augmented deuterium concentration to a central heavy water. This central heavy water plant could be a Combined Electrolysis and Catalytic Exchange (“CECE”) heavy water production plant or a Girdler Sulfide heavy water plant. The deuterium content of water at the remote sites is increased and provides water stream(s) with augmented deuterium concentration to feed to the central heavy water production plant. This could be a first stage of the central CECE deuterium enrichment plant, increasing its capacity for heavy water production approximately in the ratio of its enrichment above natural deuterium concentrations. By relatively simple utilization of available deuterium enrichment capacity at the remote sites, advantages are achieved from a larger scale of heavy water production at the central production plant. The invention further provides systems and methods for adapting chlorate and chlorine dioxide systems which produce hydrogen to additionally produce deuterium-enriched water.
Owner:ISOWATER CORP
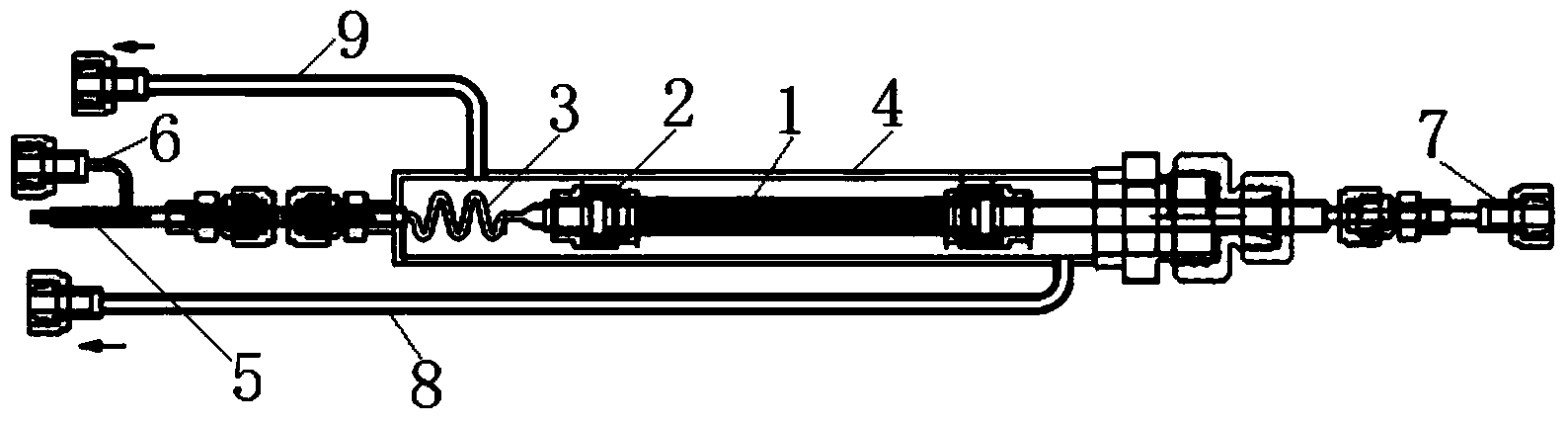
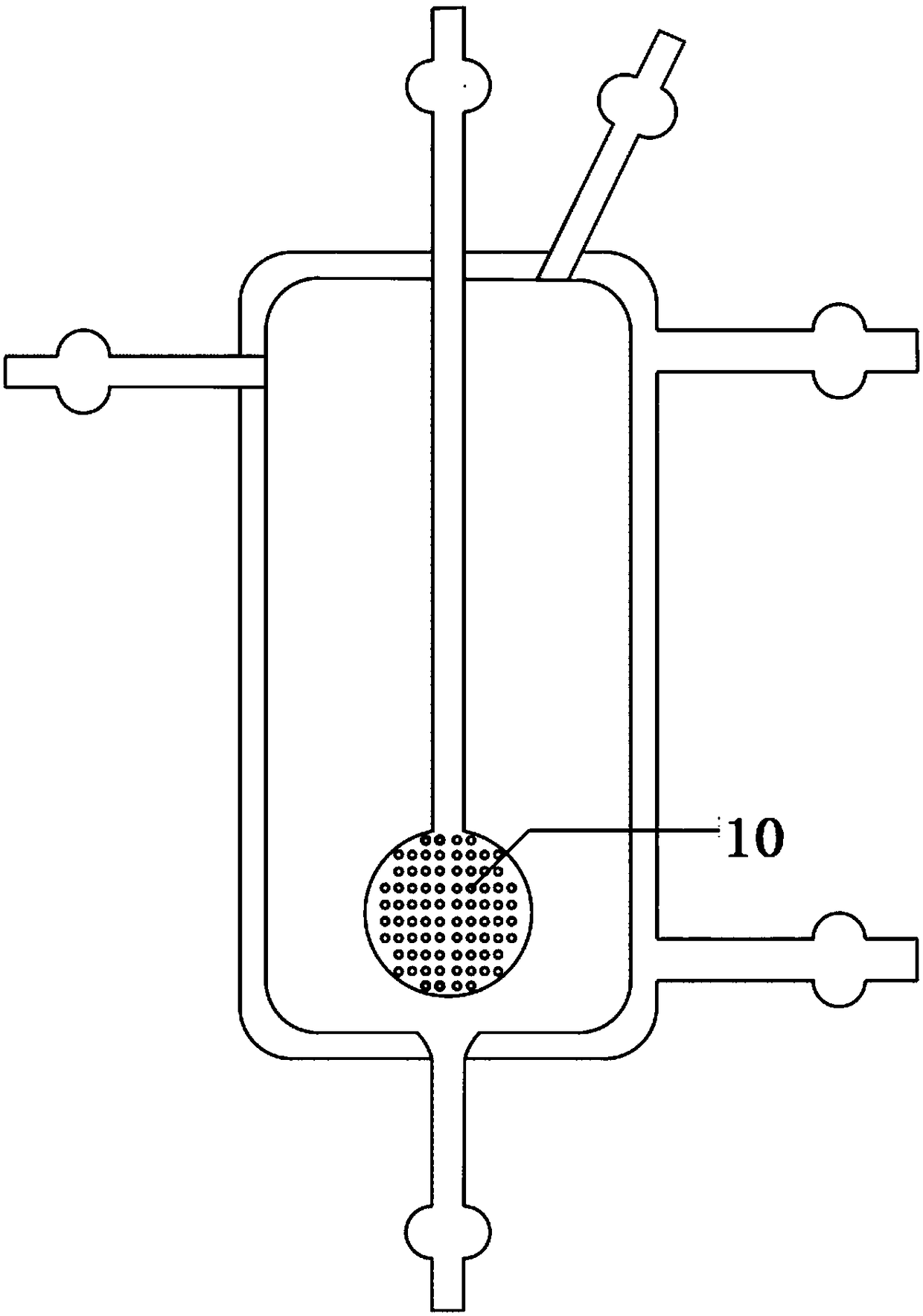
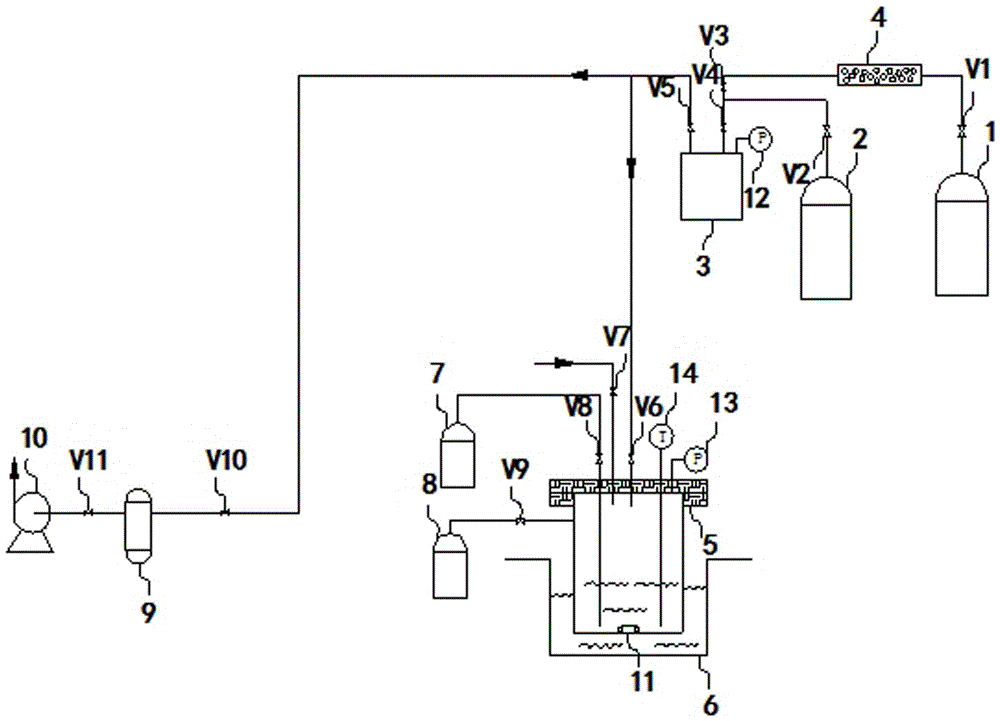
Method for measuring separation coefficient during process of silicon isotope separation (chemical exchange method) and apparatus thereof
InactiveCN105548329AReasonable structureEasy to operateMaterial analysis by electric/magnetic meansSeparation coefficientGas phase
The invention discloses a method for measuring the separation coefficient during the process of silicon isotope separation (chemical exchange method) and an apparatus thereof. According to the method, an alcohol complexing agent is adopted, SiF4 gas and silicon tetrafluoride alcohol complex carry out silicon isotope chemical exchange reactions, and silicon isotope separation coefficients under different pressures at different temperatures are measured. The apparatus comprises a nitrogen gas steel cylinder (1), a reaction gas SiF4 steel cylinder (2), a SiF4 buffer tank (3), a molecular sieve column (4), a chemical exchange reactor (5), a freezing / heating device (6) with stirring and temperature adjusting functions, a liquid phase sampling bottle (7), a gas phase sampling bottle (8), a buffer tank (9), and a vacuum pump (10). The apparatus has a reasonable structure, and the method and apparatus can be used to measure the separation coefficients of isotope exchange of a silicon tetrafluoride- silicon tetrafluoride complex system under different pressures at different temperatures.
Owner:BOHAI UNIV
Method for preparing O-nitric acid
The invention relates to a method for preparing O-nitric acid, comprising the following steps of: (1) carrying out oxygen isotope exchange reaction between nitric acid material and oxygen-18 water in a sealed reaction kettle at the temperature between 50 DEG C and 80 DEG C for 1 to 10 days; (2) introducing ammonia gas to solution obtained after reaction for neutralization and removing oxygen-18 water with reduced abundance ratio through distillation at reduced pressure to obtain O-ammonium nitrate crystals in the reaction kettle; and (3) adding excessive acid to the reaction kettle to fully dissolve the O-ammonium nitrate crystals in the reaction kettle so as to regenerate O-nitric acid, and carrying out distillation at reduced pressure again to distill the regenerated O-nitric acid into liquid nitrogen trap so as to obtain the O-nitric acid product. Compared with the prior art, the invention has the advantages of simple process, easy operation, low energy consumption, high purity of products and low loss of O and the like.
Owner:SHANGHAI RES INST OF CHEM IND +1
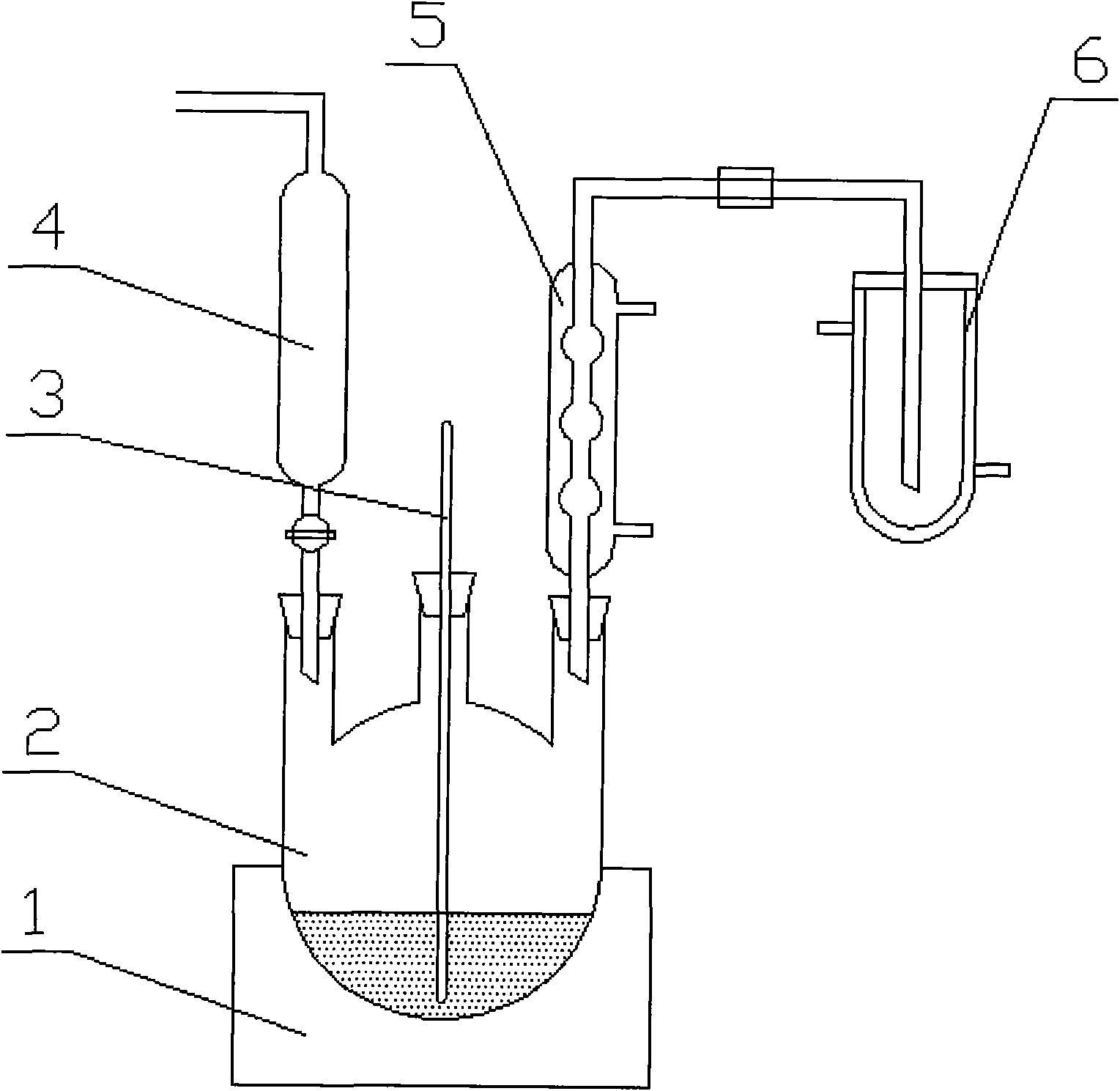
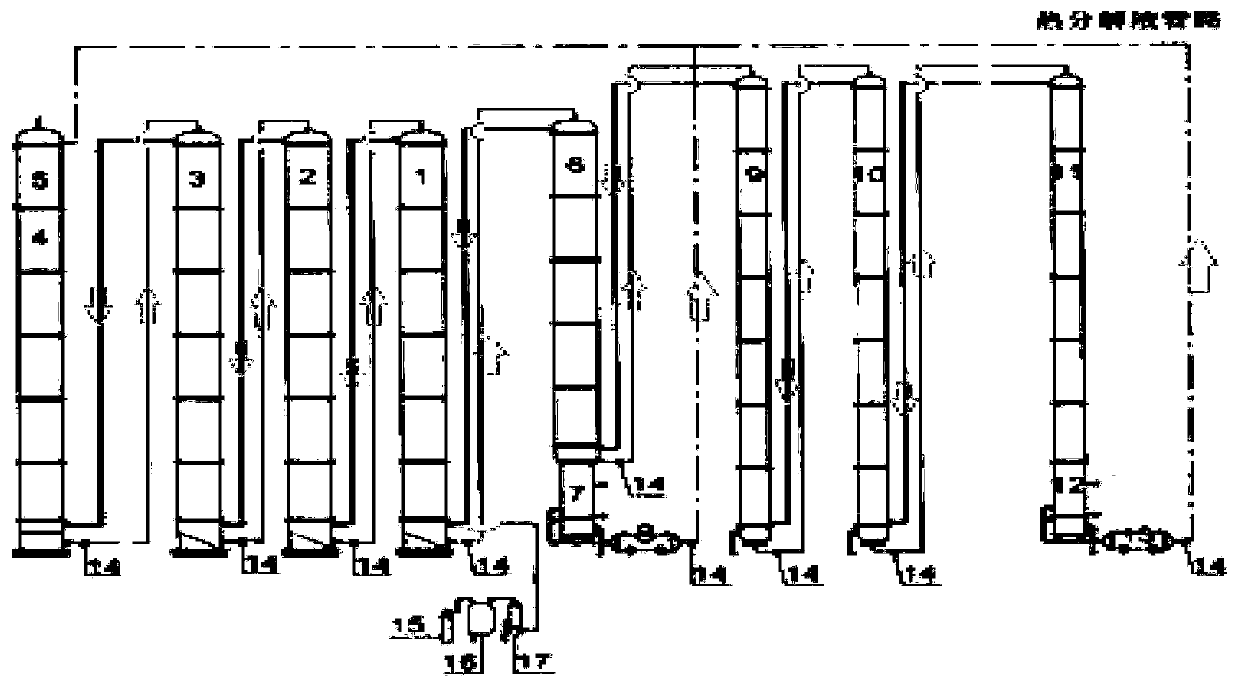
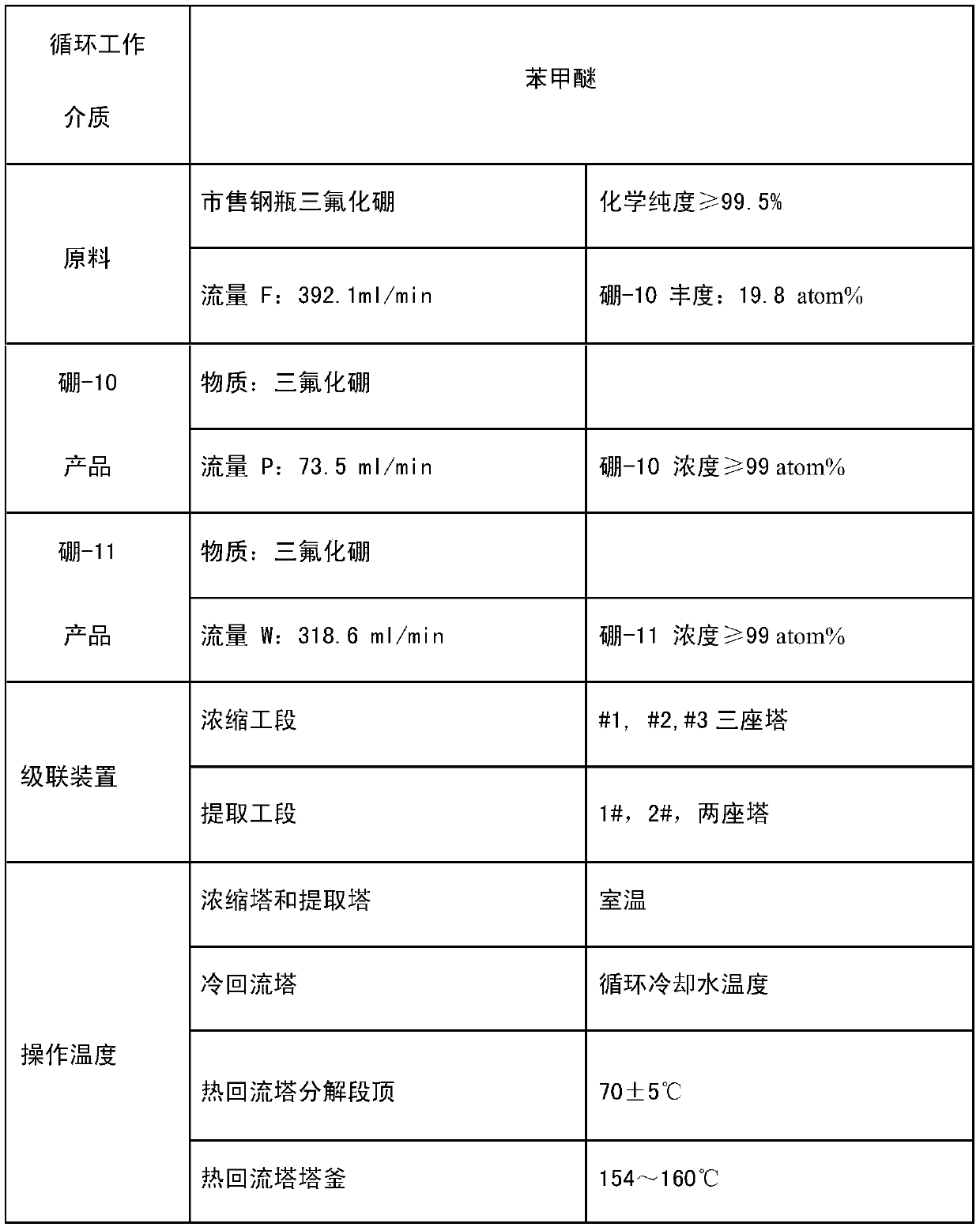
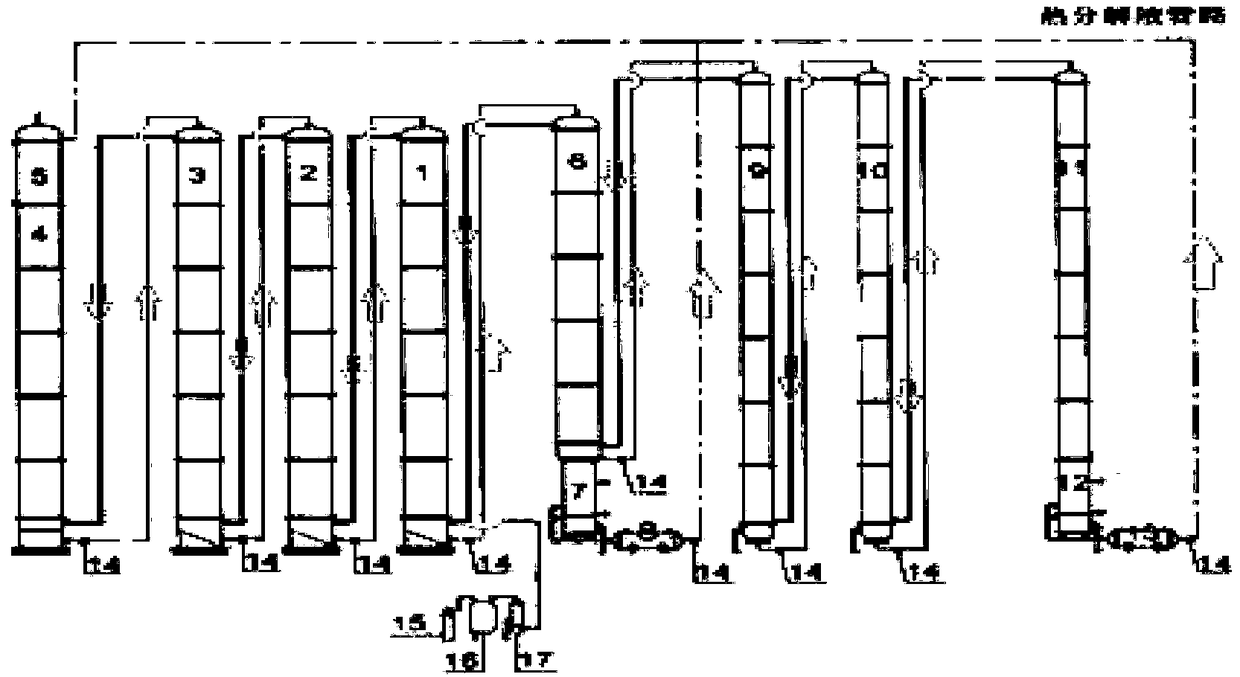
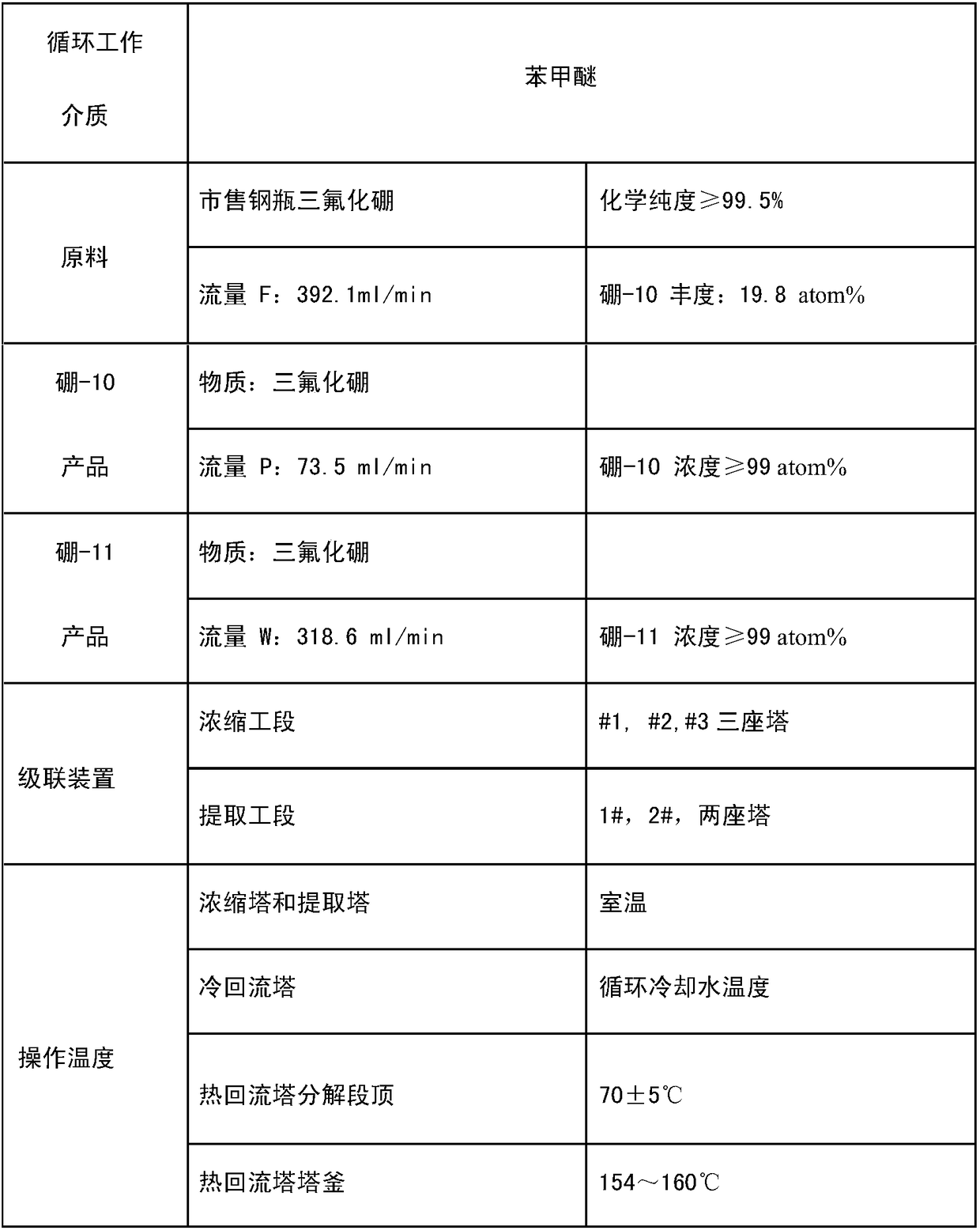
A method and system for simultaneously producing high-concentration boron-10 boron trifluoride and high-concentration boron-11 boron trifluoride
ActiveCN108275691BExchangeRealize energy saving and zero emissionBoron halidesBoron trifluoridePhysical chemistry
Owner:大方元素(广东)科技有限公司
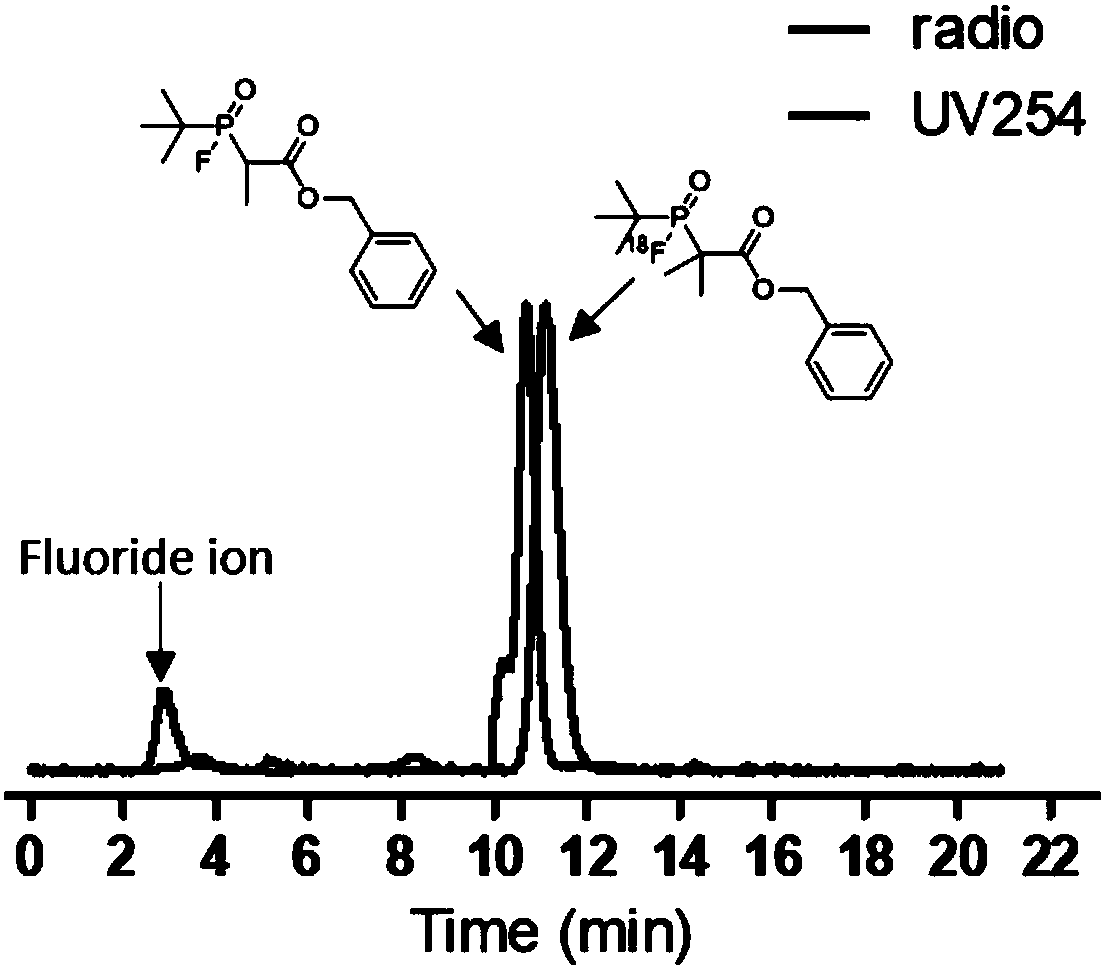
Fluorinated phosphine oxide compound and application thereof in positron emission imaging
ActiveCN108586524AQuick buildGentle buildGroup 5/15 element organic compoundsRadioactive preparation carriersImaging agentHeat sensitive
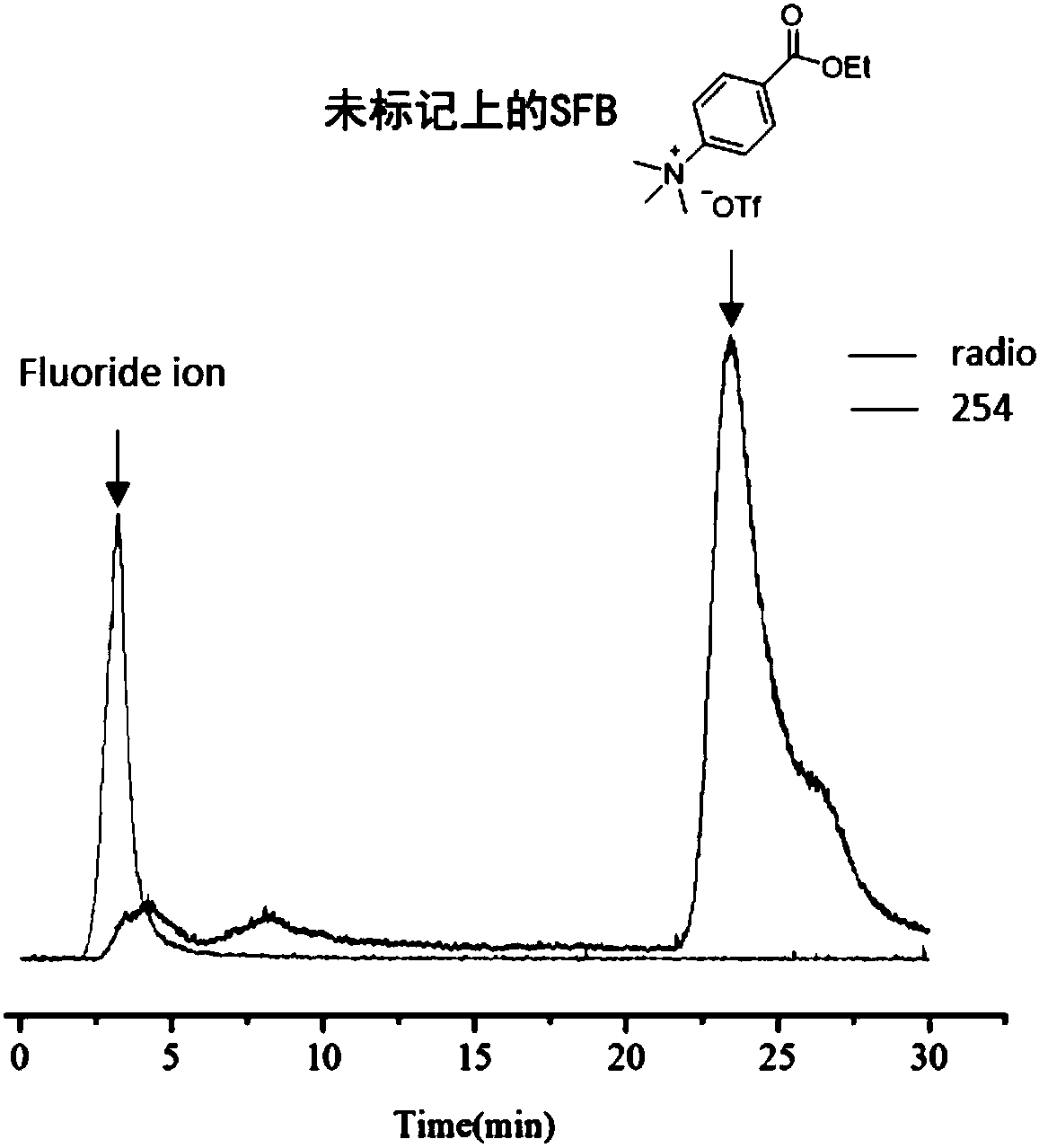
The invention relates to a fluorinated phosphine oxide compound used in the preparation of a positron emission imaging agent and a preparation method thereof, and has a structure shown in a formula I.For the first time, fluorinated phosphine oxide is used as an 18F-labeled auxiliary group, a positron nuclides probe is constructed by a 18F-19F isotope exchange strategy, a labeling method is mild in conditions, reactions can be directly conducted in an 18F-aqueous solution without blow-drying 18F-, and the compound has high radiochemical yield, easy purification, no need for high performance liquid chromatography separation and purification and broad application prospects in the field of positron drugs of preparing biomolecules such as heat sensitive and solvent sensitive peptides or proteins and the like.
Owner:XIAMEN UNIV
Isotope enrichement method
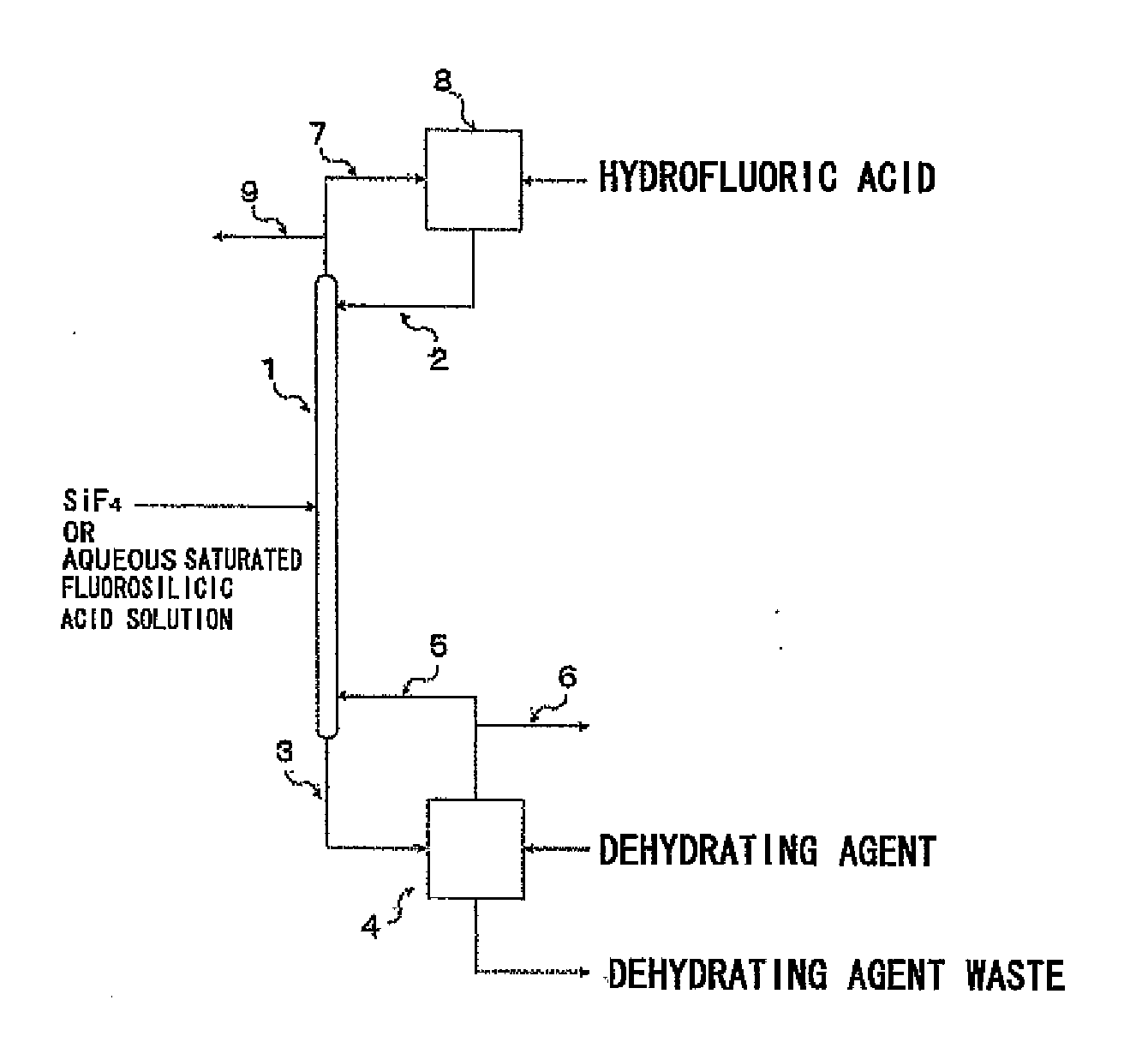
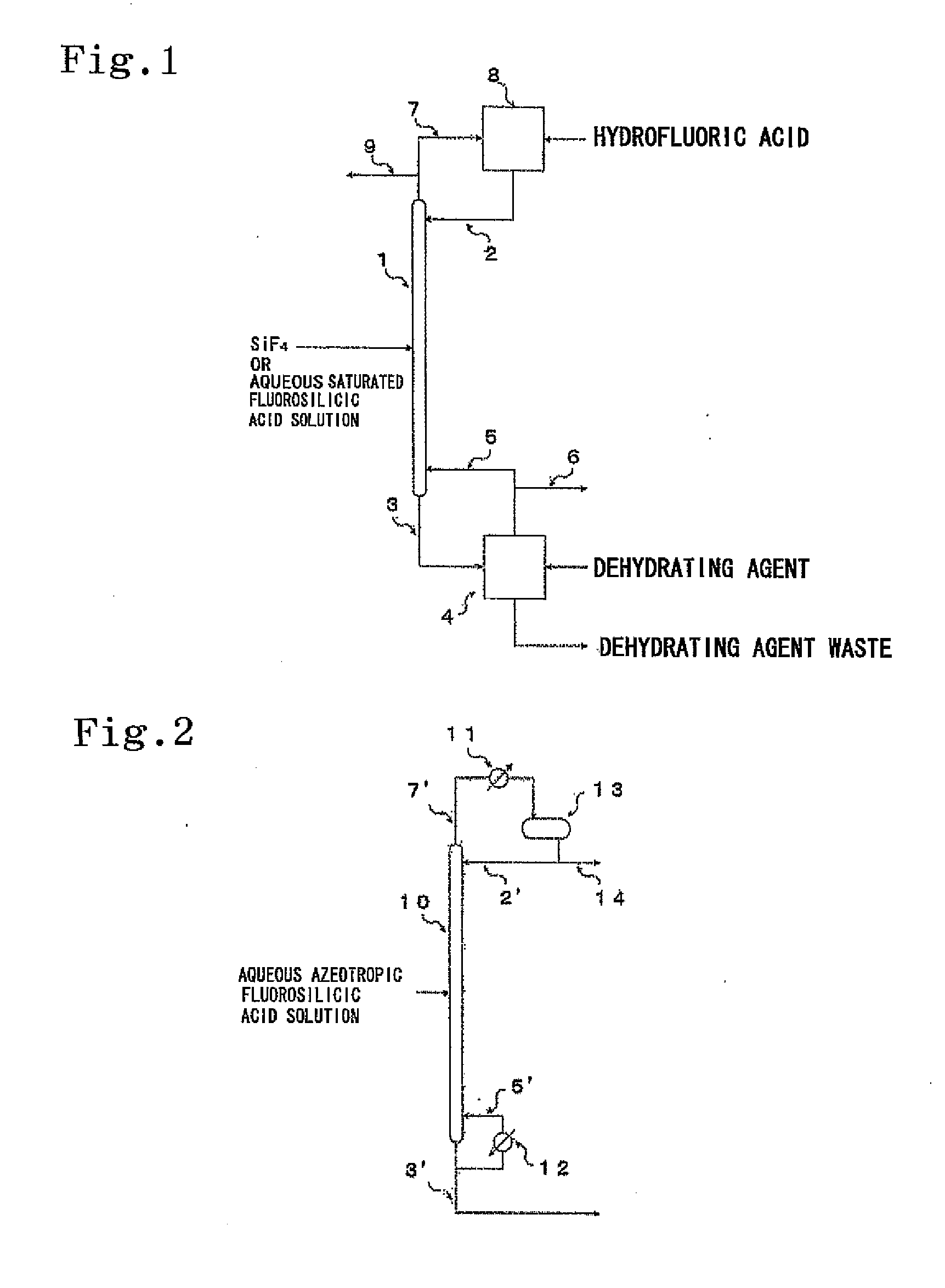
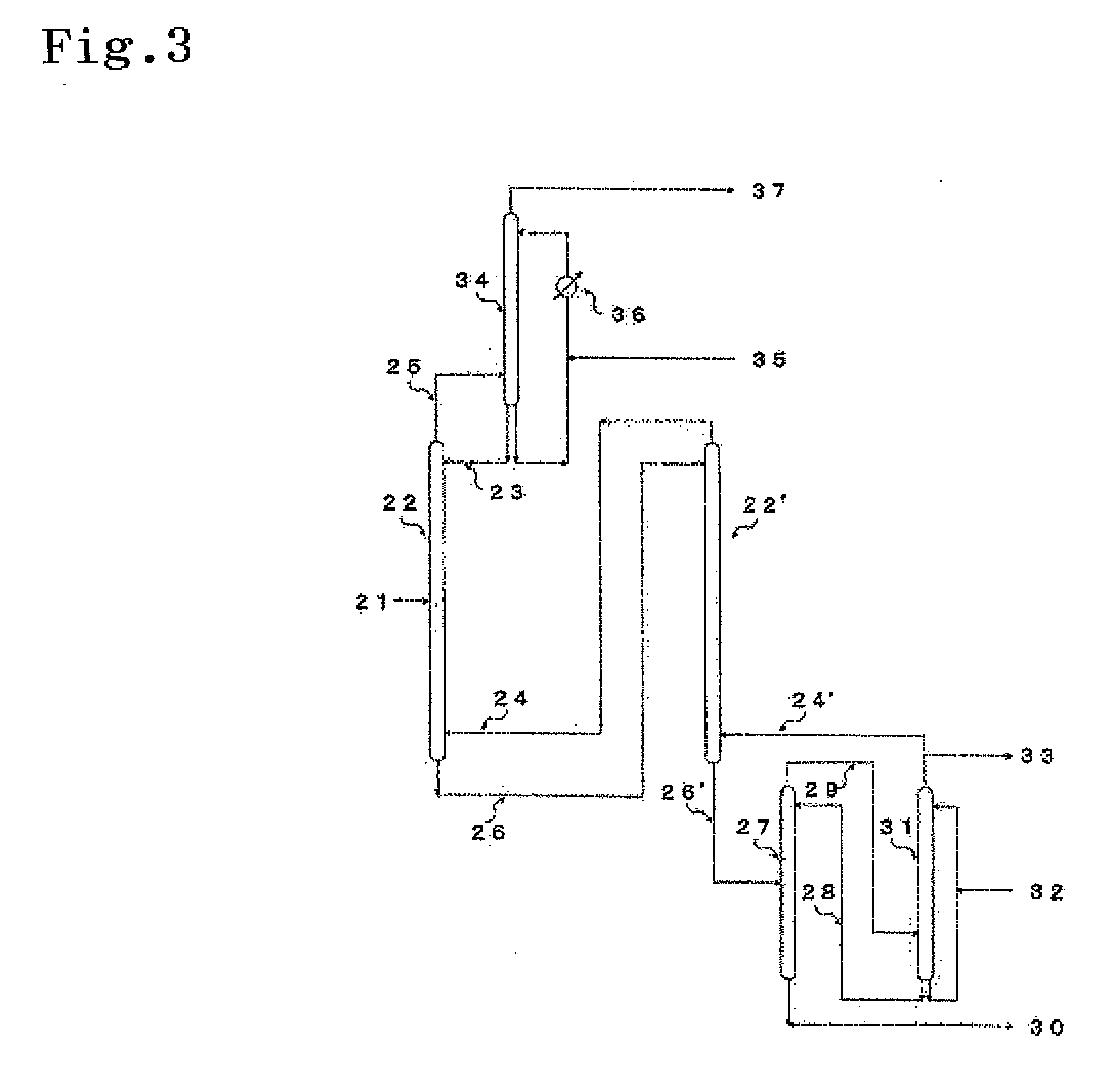
An isotope enrichment method comprising the step of performing the isotope exchange between an aqueous solution containing at least two components each represented by the formula: H2O—H2SiF6·nSiF4 (wherein n≧0) and a gas containing SiF4 to enrich a stable Si isotope.
Owner:STELLA CHEMIFA CORP
Method and system for simultaneously producing high-concentration boron-10 boron trifluoride and high-concentration boron-11 boron trifluoride
ActiveCN108275691AExchangeRealize energy saving and zero emissionBoron halidesHigh concentrationReflux
The invention discloses a method and a system for simultaneously producing high-concentration boron-10 boron trifluoride and high-concentration boron-11 boron trifluoride. With a small amount of commercially available bottled boron trifluoride as a material and a boron trifluoride / nitromethane or ether compound as a boron isotopes exchange working system, by cold-heat reflux at a concentration station and an extraction station in a cascade device, the boron trifluoride and a complex produced by the nitromethane or ether compound absorbing the boron trifluoride undego boron isotopes exchange, realizing the circulating reflux separation of boron-10 and boron-11. The system has a compact structure, is easy to operate, can be easily continuously automated, and not only greatly reduces the dosage of the boron trifluoride material, but also solves the problem of waste boron trifluoride discharge, moreover, energy consumption is reduced by about 80 percent, energy saving and zero discharge are realized, the production cost is greatly reduced, and the concentrations of the obtained boron-10 and boron-11 products are higher than or equal to 99 atom percent.
Owner:大方元素(广东)科技有限公司
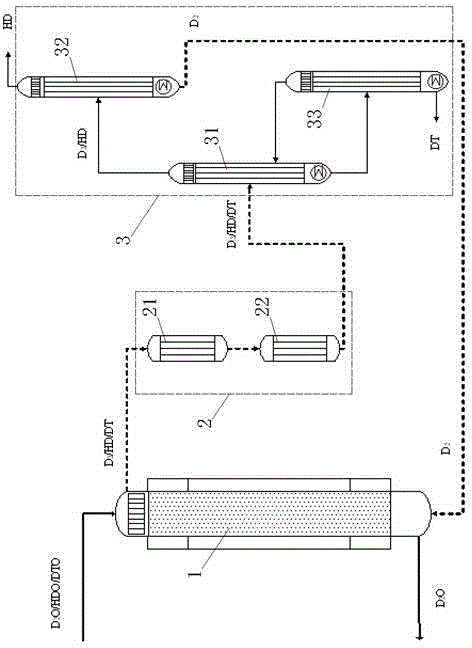
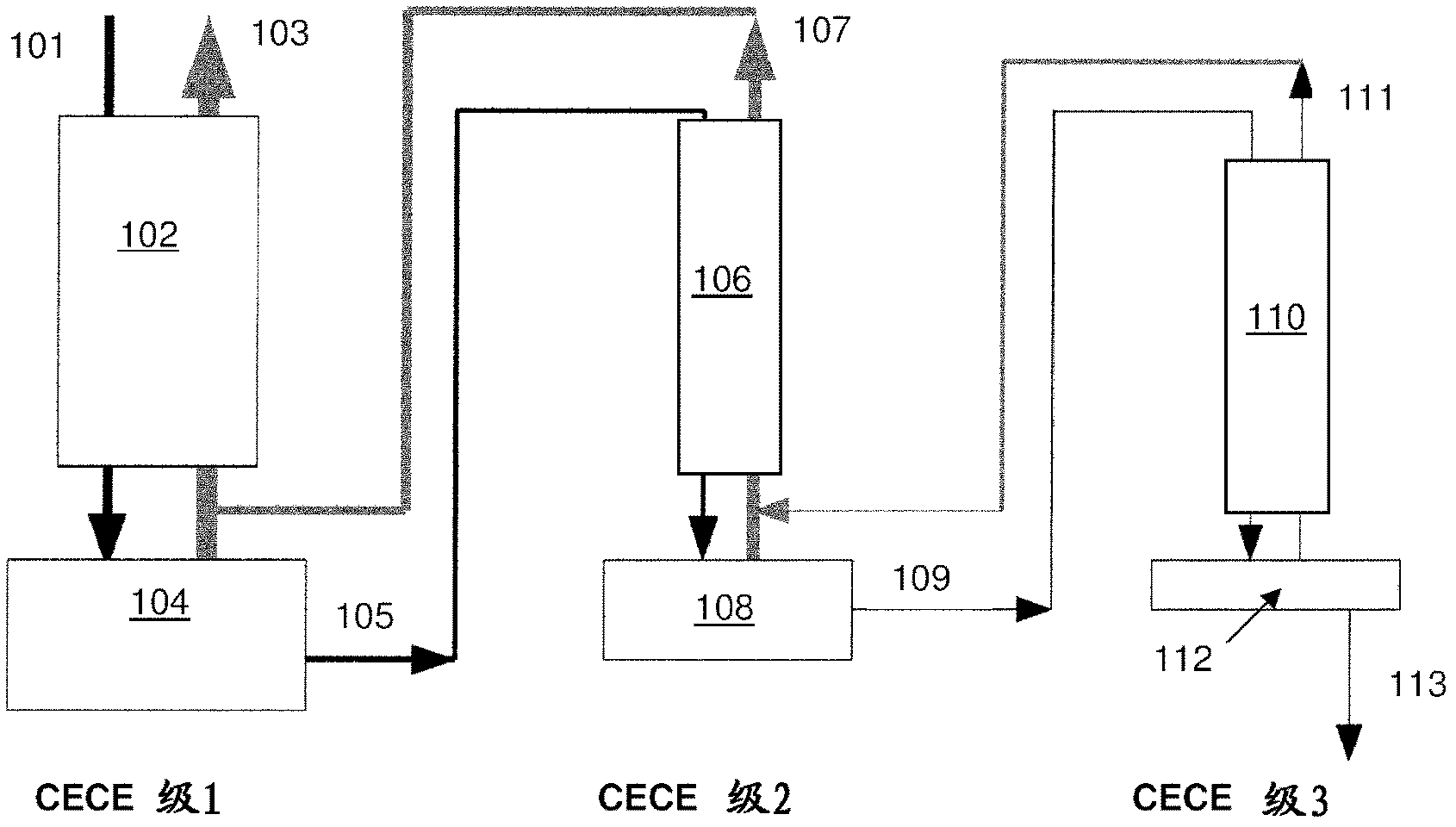
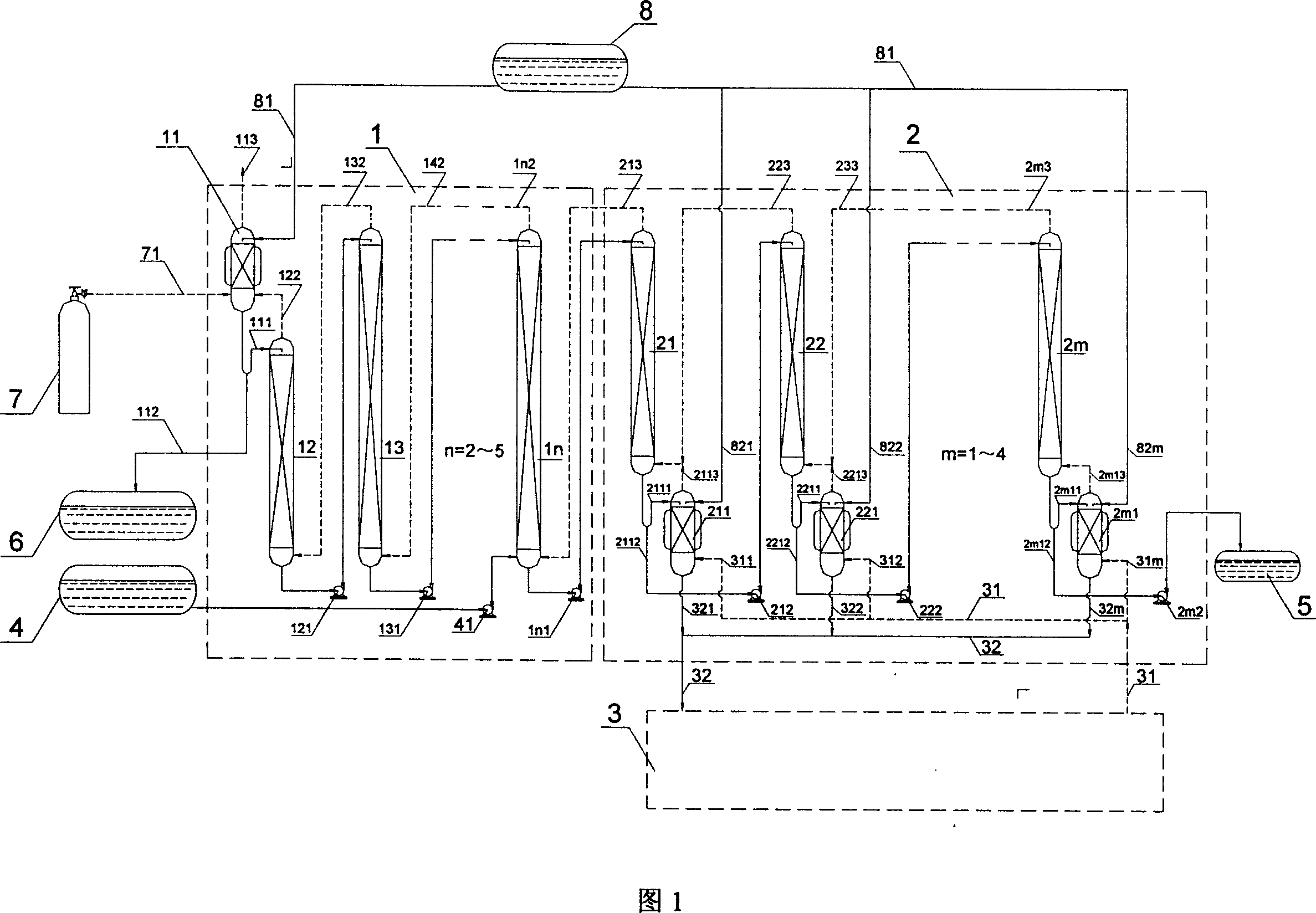
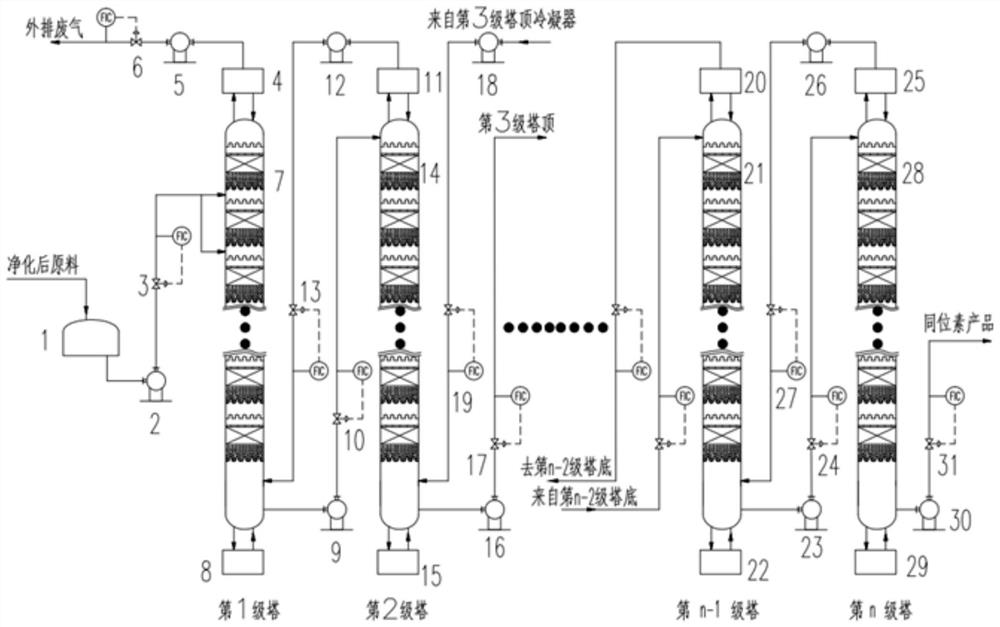
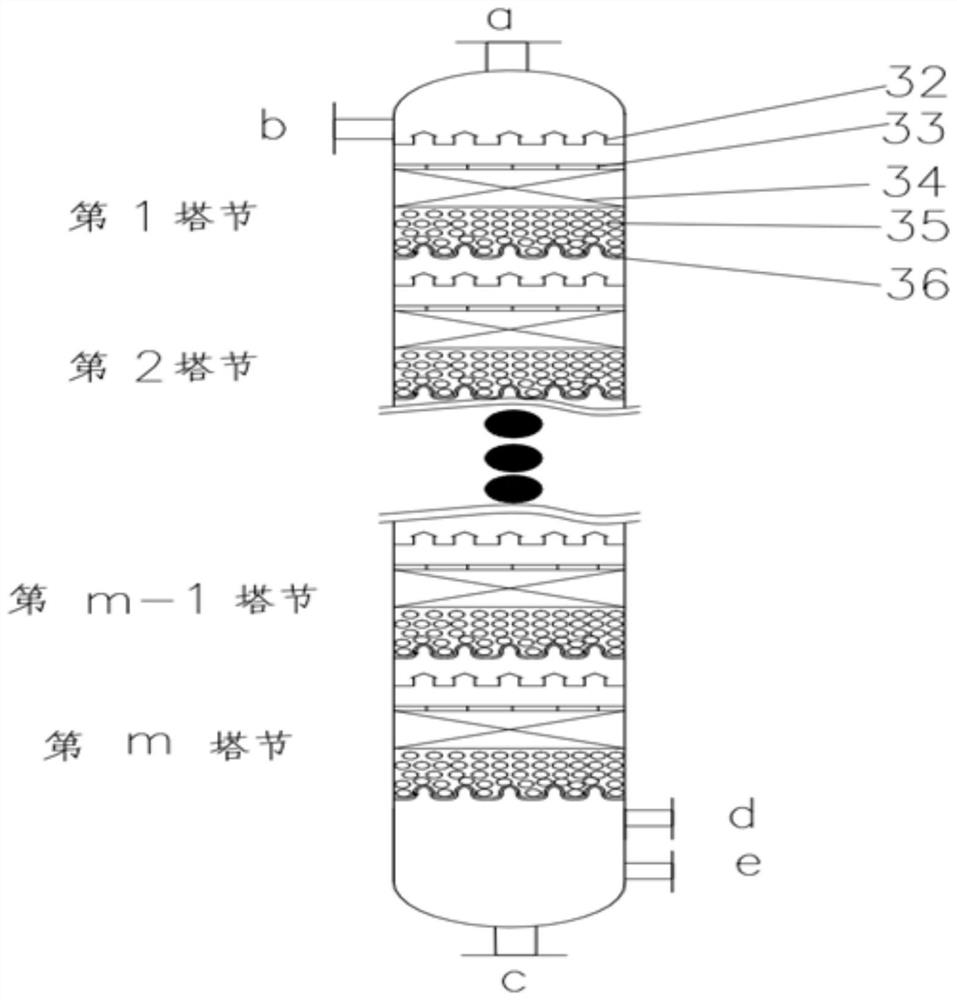
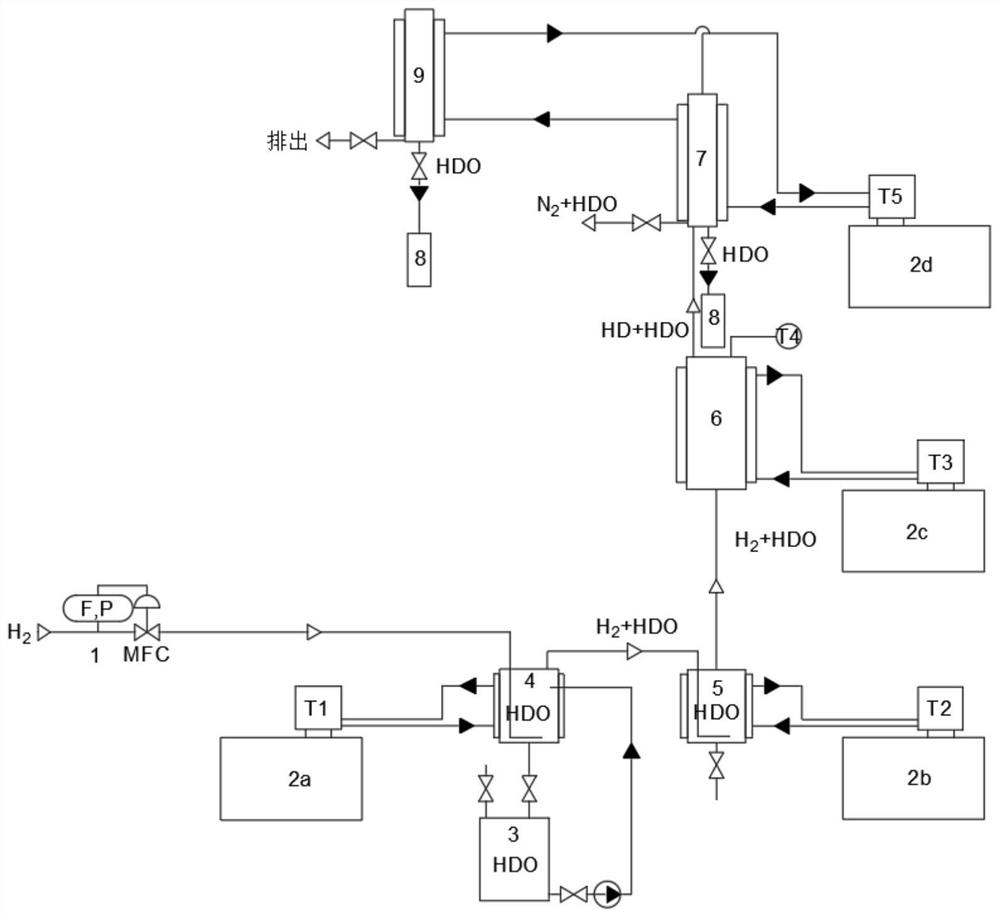
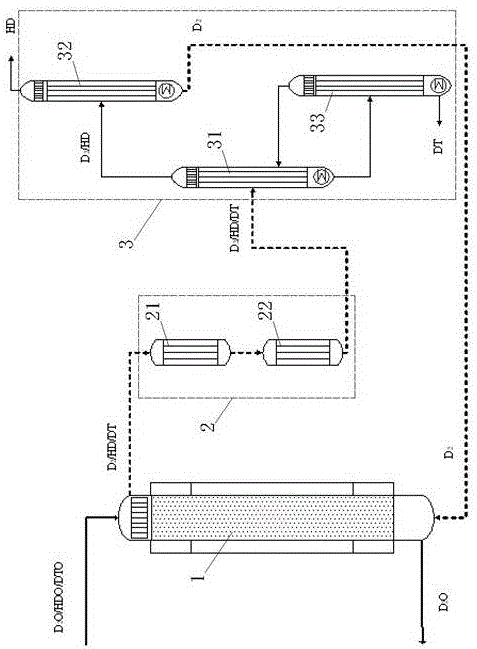
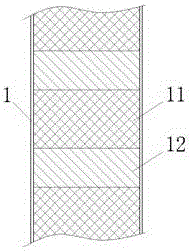
Stable isotope low-temperature separation and concentration device and technology
PendingCN112156653AOvercome the disadvantages of single rectification functionReduce the number of cascade towersIsotope separationThermodynamicsReboiler
The invention relates to a stable isotope low-temperature separation and concentration device and technology. The technology comprises the following steps that purified high-purity feed gas enters a low-temperature separation and concentration device formed by the series connection of n difunctional composite separation towers from a buffer tank through pump metering, and each tower top provides an initial liquid phase of the tower corresponding to a condenser; and each tower bottom provides an initial vapor phase of the tower corresponding to a reboiler, vapor and liquid are in countercurrentcontact in each tower, two processes of isotope exchange reforming and vapor-liquid rectification are continuously and alternately carried out, a heavy isotope product is continuously produced at thelast nth-stage tower bottom through pump metering, and waste gas subjected to heavy isotope separation is continuously produced at the first-stage tower top condenser through pump metering. The device is a cascade system formed by connecting n bifunctional composite separation towers which are horizontally arranged in series, and each bifunctional composite separation tower is composed of m towersections; and the device and the technology effectively overcome the defects of single function and the like of the separation tower in the traditional low-temperature rectification process, reduce the number of cascaded towers, simplify the process and save the energy consumption.
Owner:湖北楚儒同位素科技有限公司
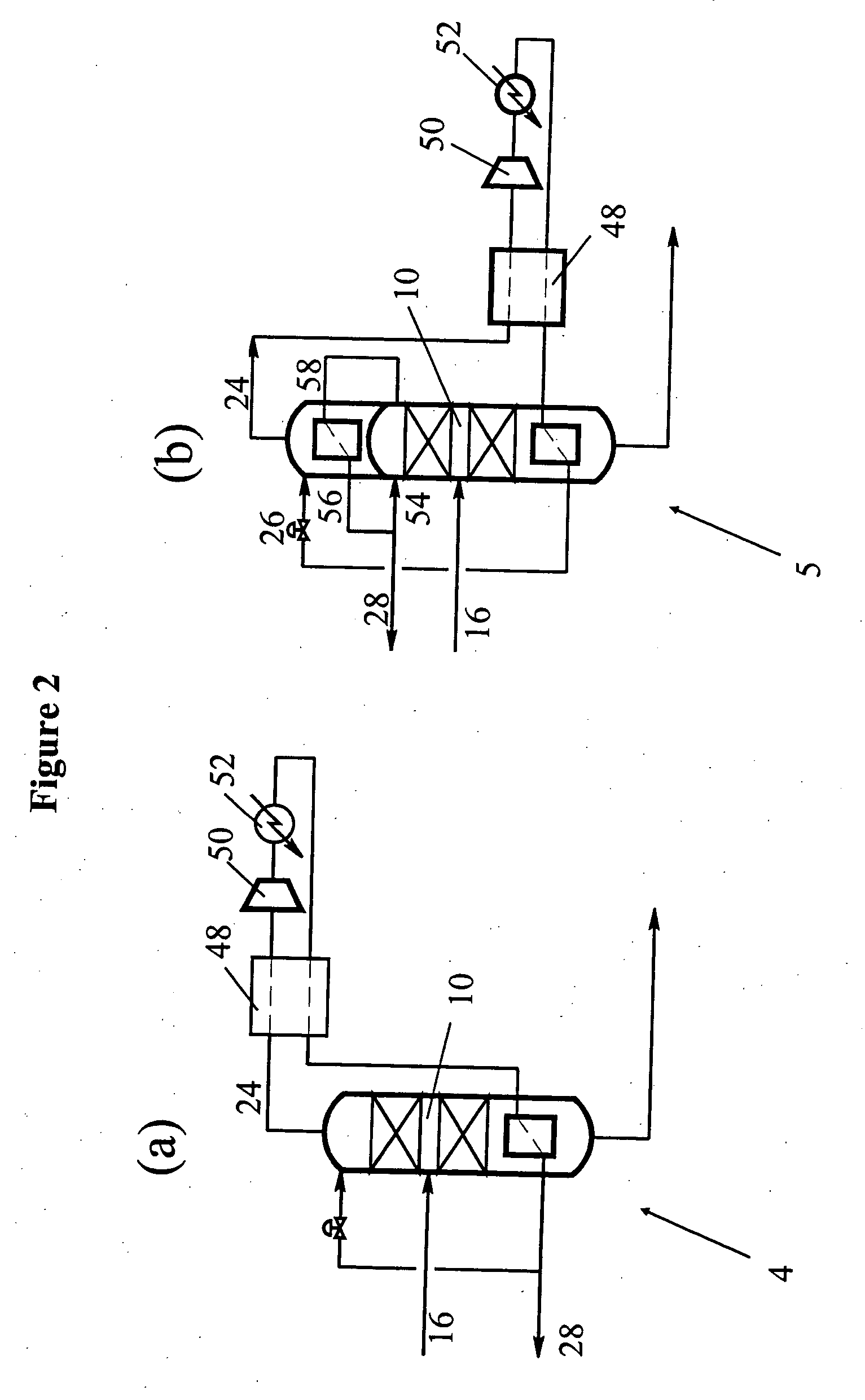
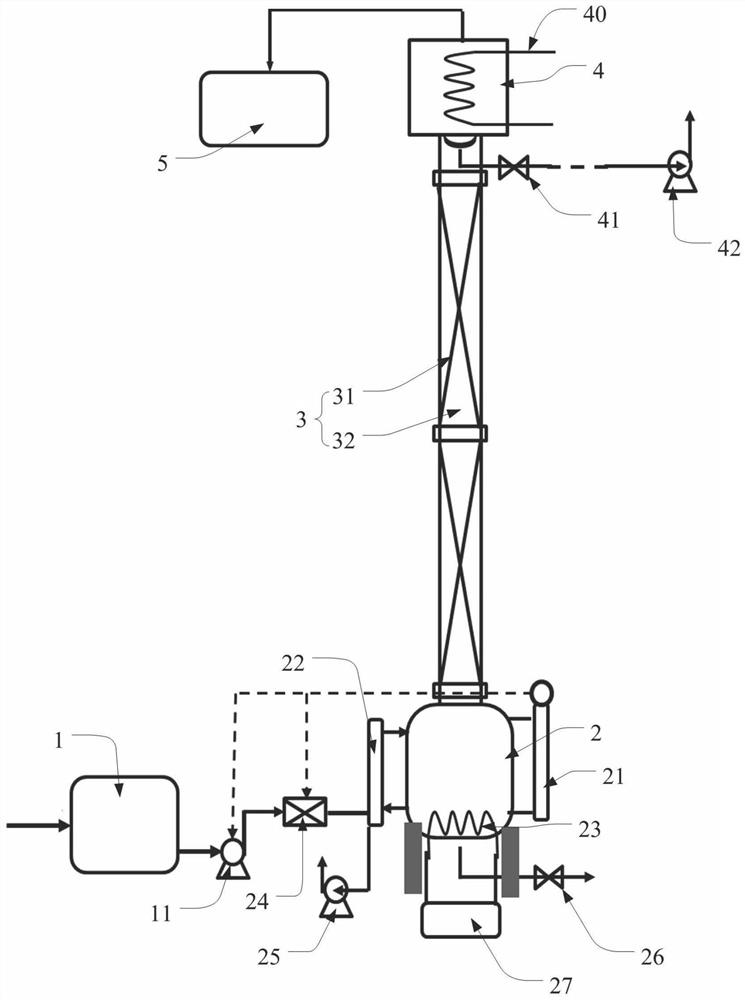
High-concentration tritium water treatment device
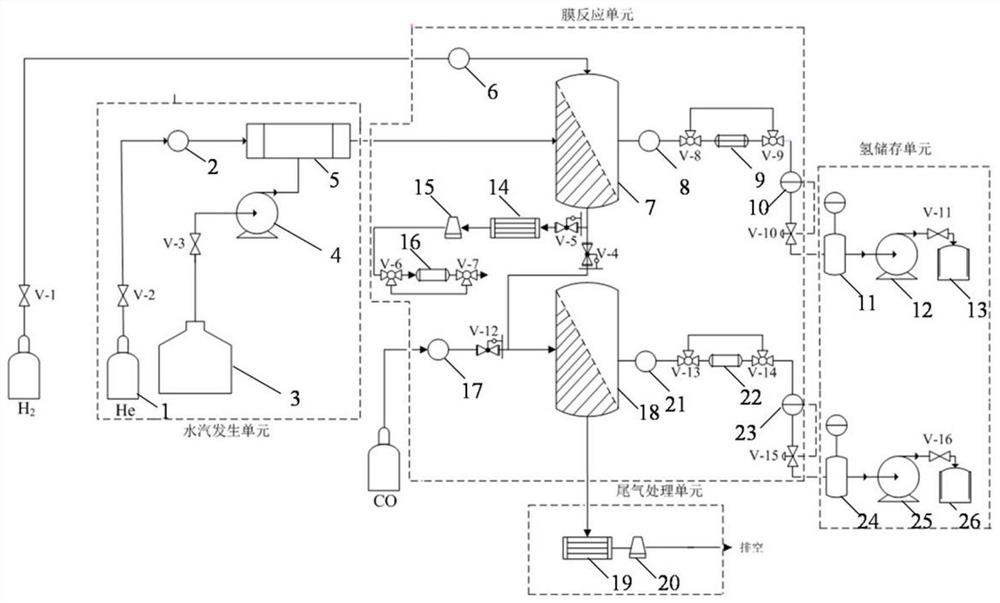
ActiveCN112037958ANuclear energy generationRadioactive decontaminationPtru catalystChemical reaction
The invention discloses a high-concentration tritium water treatment device. The high-concentration tritium water treatment device comprises a water vapor generation unit, a membrane reaction unit, ahydrogen storage unit and a tail gas treatment unit which are connected in sequence. According to the invention, the first-stage reactor adopts a Pt catalyst loaded with variable valence oxides (TiO2,CeO2 and ZrO2) is adopted to change the isotope exchange reaction process of the anti-hydrogen water from the molecular level, so that the reaction rate is increased; and the second-stage reactor isfilled with a lithium-based CO2 adsorbent, a product is removed, and chemical equilibrium is further promoted to move rightwards. According to the method, the chemical reaction rate is increased, sidereactions are reduced, products are removed to promote chemical equilibrium movement, the tritium removal factor of the tritium water treatment process is increased, efficient recovery of tritium isachieved, and the device can be applied to fusion devices and other places where high-concentration tritium water is generated.
Owner:INST OF NUCLEAR PHYSICS & CHEM CHINA ACADEMY OF
A kind of ultra-light water preparation device and its preparation method
ActiveCN112357883BReduce manufacturing costExtended service lifeChemical industryIsotope separationLiquid waterTap water
The invention discloses an ultra-light water preparation device and a preparation method thereof, wherein the ultra-light water preparation device includes a water purification device for filtering raw water, a water purification device for heating and evaporating the filtered purified water The evaporator unit for the isotope exchange of the upward steam in the column with the downward reflux liquid, the distillation column for changing the vapor into liquid water and causing part to be extracted as ultra-light water, partly forming the reflux liquid The condenser and pressure control device, the water purification device is connected to the water inlet end of the evaporator device, the evaporator device, the distillation tower, and the condenser are connected in sequence, and the pressure control device is connected to the condenser to make the equipment compact and occupy The land area is small. Tap water can be used as raw water for purification treatment, which greatly reduces the production cost of ultra-light water, helps to improve the isotope separation effect, and prolongs the service life of the evaporator device. It has the advantages of convenient connection, simple structure, and energy saving Easy to operate and so on.
Owner:SHANGHAI INST OF APPLIED PHYSICS - CHINESE ACAD OF SCI
Hydrogen isotope gas phase exchange hydrophobic catalyst activity evaluation device and evaluation method
ActiveCN108802267BTargetedEasy to operateChemical analysis using catalysisTemperature controlPtru catalyst
The invention provides a device and a method for evaluating activity of a hydrophobic catalyst through hydrogen isotope gas phase exchange. The device comprises a gas control unit, a water saturationunit, a catalytic reaction system, a sample collection unit and a temperature control system, wherein the water saturation unit comprises a primary water saturator and a secondary water saturator; thecatalytic reaction system comprises a reaction pipe; the sample collection unit comprises a condensation pipe for carrying out condensed collection on water samples containing deuterium and a cold trap for carrying out liquid nitrogen cooling and collection on the water samples containing the deuterium; the temperature control system consists of four independent temperature control units which are respectively used for controlling temperatures of the primary water saturator, the secondary water saturator, the reaction pipe and the condensation pipe. According to the device and the method disclosed by the invention, the hydrogen gas passes through the saturated deuterium-containing water, and performs gaseous hydrogen isotope exchange reaction on the catalyst bed, the deuterium-containingwater is collected by adopting backflow condensation and / or cold trapping, so that the content of the deuterium in the water before exchange is quantitatively analyzed through an infrared method, andthe catalytic performance of the catalyst is evaluated. The device and the method have the advantages that an operation is simple, and the device and the method are suitable for evaluating the performance of the catalyst in the experiment and research stage.
Owner:HARBIN ENG UNIV
Simultaneous upgrade of heavy water and detritium process
ActiveCN104828778BSimple processLow one-time investment costHeavy waterIsotope exchangeGenus Protium
The invention discloses a simultaneous upgrading and tritium removal process of heavy water, and belongs to the technical fields of heavy water upgrading and tritium removal. The simultaneous upgrading and tritium removal process of heavy water uses Pt / C / PTFE or Pt-SDB as a catalyst, and employs D2 to conduct isotope exchange with HDO and DTO in heavy water. The process provided by the invention avoids disadvantages of two separate devices in the traditional process for protium and tritium removal in heavy water, achieves simultaneous removal of protium and tritium, effectively reduces production cost and risks in the treatment process; in addition, by identifying key process parameters, the reaction effect of the upgrading and tritium removal process is ensured, effect of each treatment process is improved, and the economic performance of the whole process is improved.
Owner:INST OF NUCLEAR PHYSICS & CHEM CHINA ACADEMY OF
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com