Method for preparing O-nitric acid
A technology of nitric acid and ammonium nitrate, applied in the direction of nitric acid, nitrogen oxides/oxyacids, etc., can solve the problem of a large increase in the consumption of heavy oxygen water, and achieve the effects of less 18O loss, high product purity, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
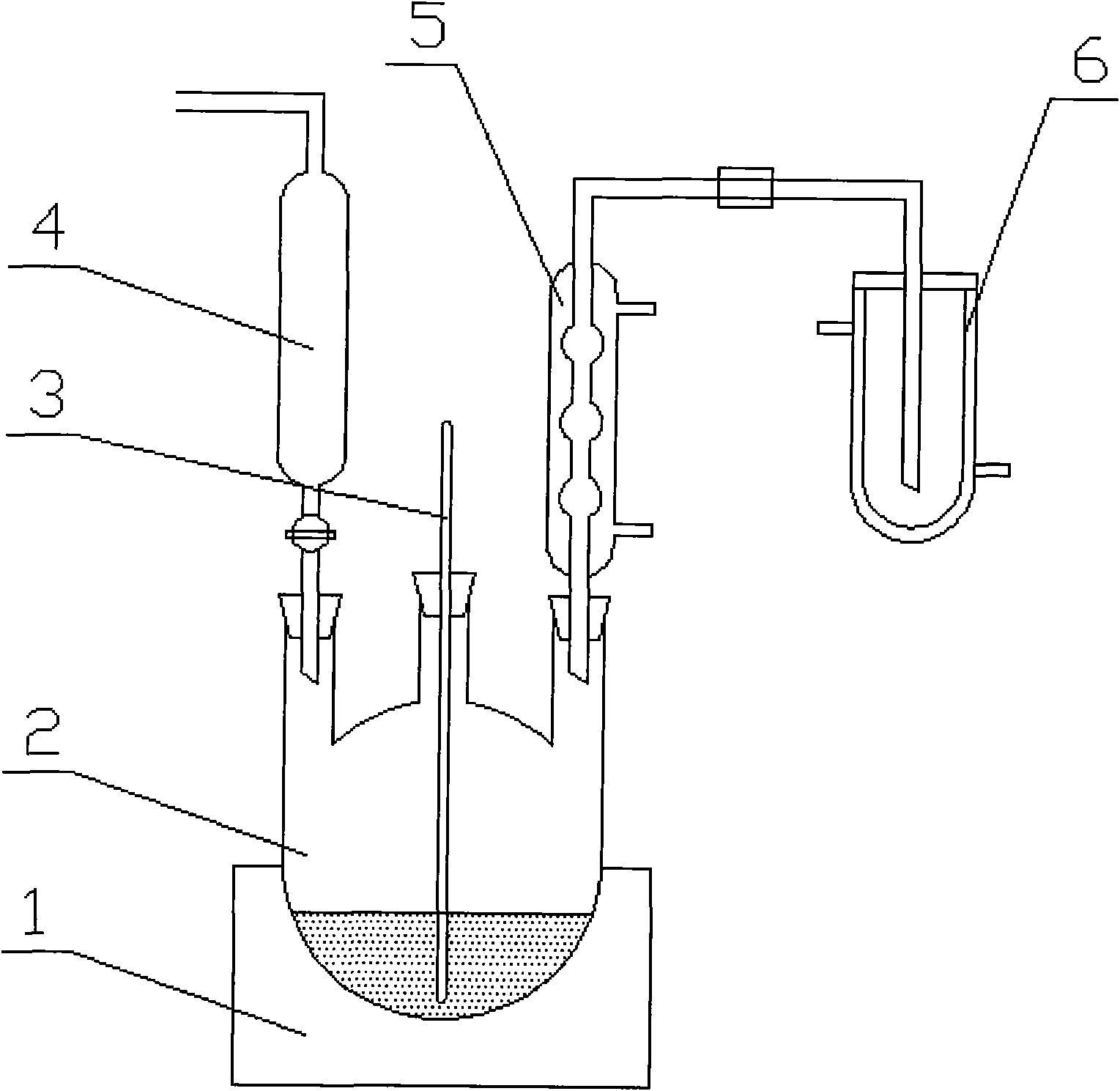
Image
Examples
Embodiment 1
[0026] Will 18 O abundance is 0.2% nitric acid 2mol and oxygen-18 water 25mol ( 18 O abundance is 98.5%) carry out the exchange reaction of oxygen isotope in the reaction kettle, the temperature is controlled at 70 ℃, the whole reaction kettle is kept in a closed state, and the reaction time is 10 days. In the reacted solution, excessive ammonia gas was passed to neutralize it, and the oxygen-18 water was distilled off under reduced pressure. The pressure was controlled at 80mmHg, and the temperature was controlled at 40-42°C. 18 O-ammonium nitrate crystals. Add excess methanesulfonic acid in the reactor, fully dissolve the 18 O-ammonium nitrate crystals, regenerated 18 O-nitric acid, carry out vacuum distillation again, pressure is controlled at 80mmHg, temperature is controlled at 40~42 ℃, will generate 18 O-nitric acid was evaporated into a liquid nitrogen cold trap to obtain 18 O abundance is 16% 18 O-nitric acid product 0.8mol.
Embodiment 2
[0028] Will 18 O abundance is 39% nitric acid 1.2mol and oxygen-18 water 10mol ( 18 O abundance is 98.5%) carry out the exchange reaction of oxygen isotope in the reaction kettle, the temperature is controlled at 70 ℃, the whole reaction kettle is kept in a closed state, and the reaction time is 6 days. In the solution after reaction, excess ammonia gas was passed to neutralize, and oxygen-18 water was distilled off under reduced pressure. The pressure was controlled at 100mmHg, and the temperature was controlled at 48-52°C. 18 O-ammonium nitrate crystals. Add excess hydrochloric acid in the reactor, fully dissolve the 18 O-ammonium nitrate crystals, regenerated 18 O-nitric acid, carry out vacuum distillation again, pressure is controlled at 100mmHg, temperature is controlled at 48~52 ℃, will generate 18 O-nitric acid was evaporated into a liquid nitrogen cold trap to obtain 18 O abundance is 76% 18 O-nitric acid product 0.6mol.
Embodiment 3
[0030] A sort of 18 The preparation method of O-nitric acid, this preparation method specifically comprises the following steps:
[0031] (1) After the isotope exchange reaction 18 O-nitric acid feedstock with 18 Oxygen-18 water with O abundance > 98% undergoes an oxygen isotope exchange reaction in the reactor, the 18 O-nitric acid feedstock 18 The atomic percentage of O abundance is 98%, the temperature is controlled at 50°C, the entire reactor is kept in a closed state, the reaction time is controlled at 10 days, and the molar ratio of nitric acid to oxygen-18 water is 1:20;
[0032] (2) Pass ammonia gas into the solution after the reaction to neutralize, distill under reduced pressure to remove the oxygen-18 water whose abundance has been reduced, control the pressure at 50mmHg, and control the temperature at 70°C to obtain 18 O-ammonium nitrate crystals;
[0033] (3) Add excessive trifluoroacetic acid in the reactor, fully dissolve the 18 O-ammonium nitrate crystals, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com