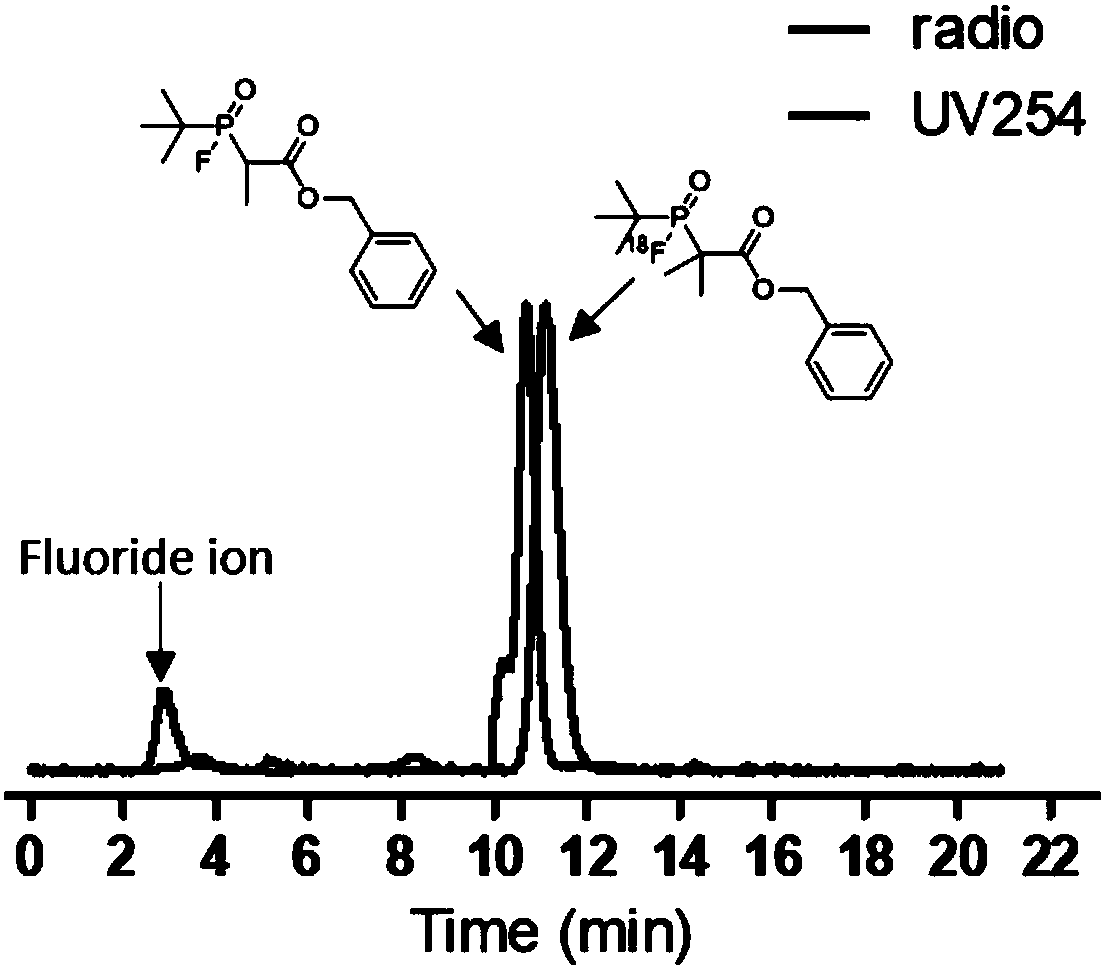
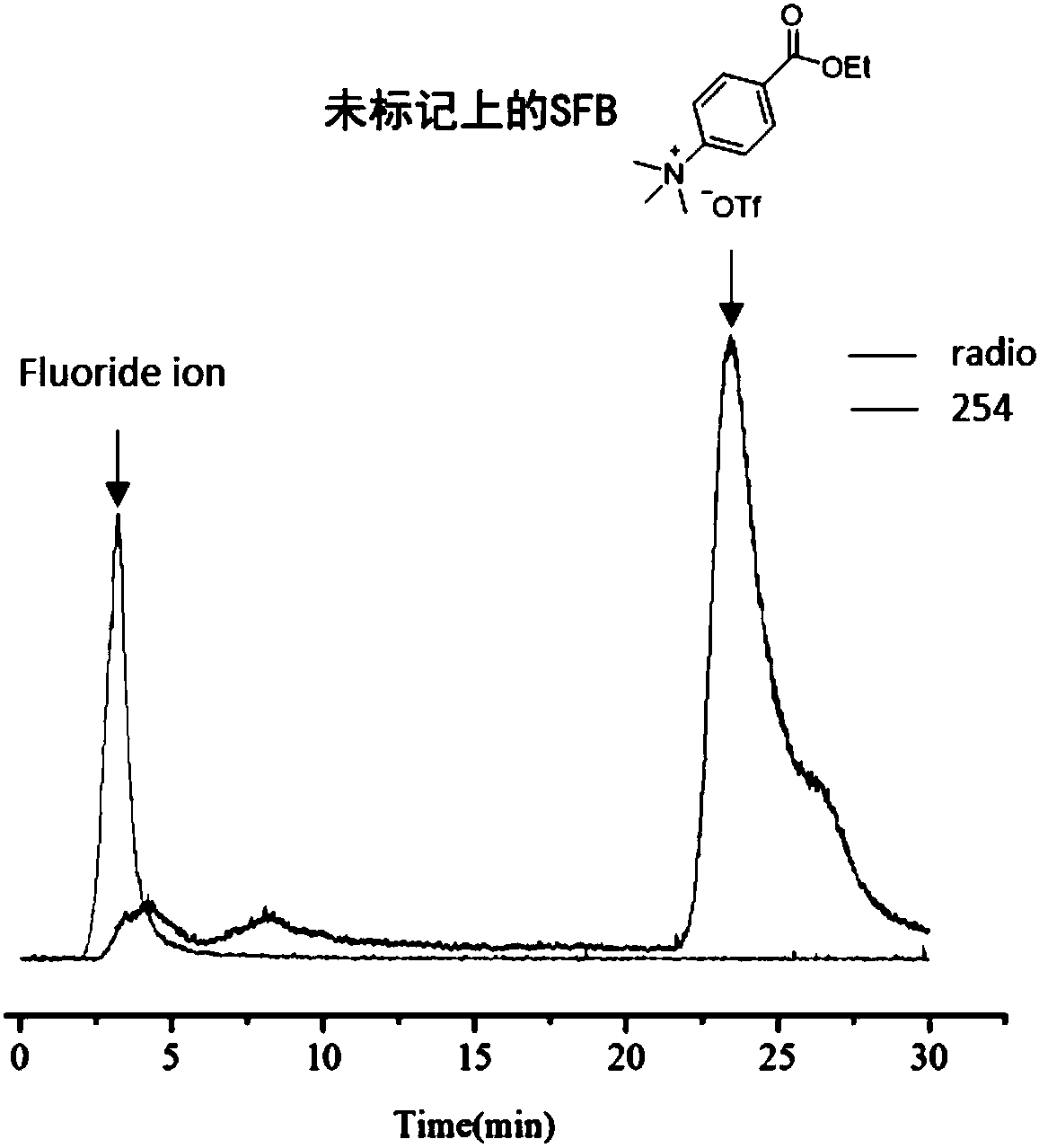
Fluorinated phosphine oxide compound and application thereof in positron emission imaging
A technology for positron emission and phosphine oxide substitution, which is applied in the direction of isotope introduction into organic compounds, compounds of Group 5/15 elements of the periodic table, luminescent materials, etc., which can solve the problems of high labeling reaction temperature, high radiochemical yield, and large molecular weight and other problems, to achieve high targeting, convenient post-processing, and low molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] This embodiment provides a positron emission imaging 18 The fluorinated phosphine oxide compound of F label, its preparation process and concrete steps are as follows:
[0078]
[0079] Tetrahydrofuran: Tetrahydrofuran DMF: N,N-Dimethylformamide
[0080] MeOH: methanol CsF: cesium fluoride
[0081] Pd / C: palladium carbon CuCl2: copper chloride
[0082] DCC: Dicyclohexylcarbodiimide 18 f - a.q.: accelerator shooting water
[0083] Synthetic steps of compound 1:
[0084] a: Take 7.5mmol of zinc powder in a dry reaction flask, add 5mL of tetrahydrofuran under the protection of argon. 1.2mmolI 2 After dissolving in 2 mL of tetrahydrofuran, add to the reaction flask. Then take 8mmol 2-bromoisobutyrate benzyl ester and dissolve it in 3mL tetrahydrofuran, then add it into the reaction flask. After the zinc powder has completely reacted, under an ice-water bath, dissolve 8 mmol of tert-butylphosphorus dichloride in 5 mL of tetrahydrofuran, slowly add it into the rea...
Embodiment 2
[0099]
[0100] Synthetic steps of compound 1:
[0101] a: Take 7.5mmol of zinc powder in a dry reaction flask, add 5mL of tetrahydrofuran under the protection of argon. 1.2mmolI 2 After dissolving in 2 mL of tetrahydrofuran, add to the reaction flask. Then take 8mmol 2-bromoisobutyrate benzyl ester and dissolve it in 3mL tetrahydrofuran, then add it into the reaction flask. After the zinc powder has completely reacted, under an ice-water bath, dissolve 8 mmol of tert-butylphosphorus dichloride in 5 mL of tetrahydrofuran, slowly add it into the reaction flask, and react overnight at room temperature. Recovery treatment: Add 20 mL of 0.1 mol / L dilute hydrochloric acid under ice-water bath, extract with ethyl acetate, spin dry and separate through column; Compound 1 is obtained.
[0102] Compound 1 NMR results: 1 H NMR (600MHz, CDCl 3 )δ1.14(d,9H,J=16.35Hz); 1.55(s,3H); 1.62(d,6H,J 1 =12.38Hz,J 2 =14.47Hz); 5.14(d,2H,J 1 =12.28Hz,J 2 = 12.28Hz); 6.51(d,1H,J=464.93Hz)...
Embodiment 3
[0110]
[0111] Synthetic steps of compound 8:
[0112] Take 2mmol of diethyl phosphite in a dry reaction bottle, add 3ml of tetrahydrofuran under the protection of argon, place it at -80°C for half an hour, add 6mmol of isopropylmagnesium bromide, and react at -80°C for 2 hours React at room temperature for 3 hours, add dilute hydrochloric acid to quench the reaction, extract with ethyl acetate, and finally spin dry and separate through the column.
[0113] Compound 8 NMR results: 1 H NMR (400MHz, CDCl 3 )δ1.24(d, 9H, J=39.46Hz); 2.03(d, 2H, J=32.51Hz); 6.33(d, 1H, J=431.69Hz). 31 P NMR (400MHz, CDCl 3 ) δ58.60(d,1P,J=464.53Hz).
[0114] Synthetic steps of compound 9:
[0115] 2mmol CsF and 2mmol CuCl 2 In the dried reaction flask, under the protection of argon, 1 mL of tetrahydrofuran was added. Subsequently, 1 mmol of product 1 was taken, dissolved in 1 mL of anhydrous tetrahydrofuran, added to the reaction flask, reacted at room temperature for 2 hours, spin-drie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com