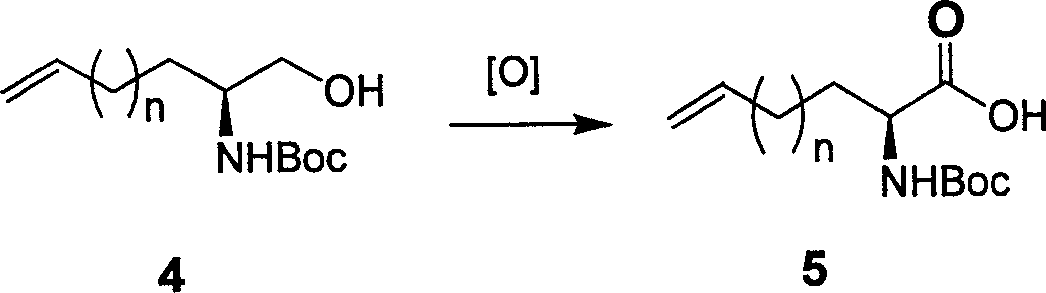Preparing process of optically active long chain N-Boc-amino acid with end olefinic bond
An optically active and amino acid technology, applied in the field of amino acid preparation, to achieve the effects of cheap and easy-to-obtain raw materials, shorten the synthesis process, and reduce costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
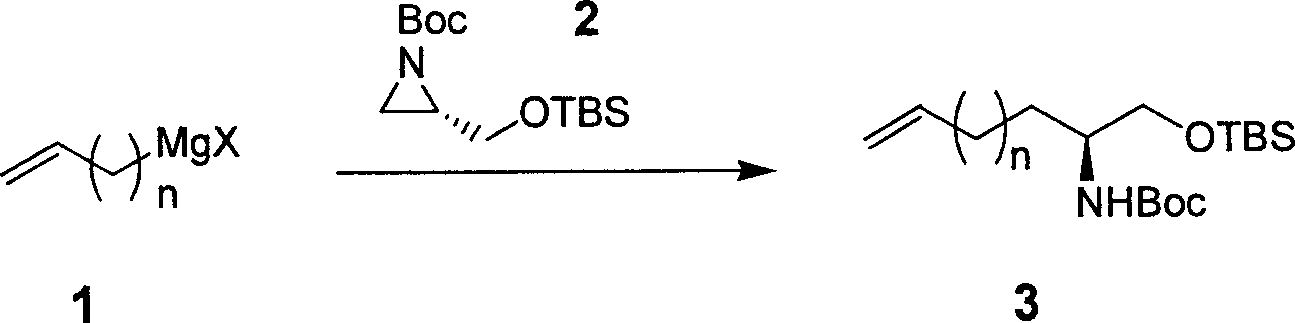
[0039] 1. Synthesis of 2-Boc-amino-4-pentenoic acid
[0040] Step 1: Synthesis of O-TBS-2-Boc-amino-4-pentenol
[0041] CuBrSMe 2 (214mg, 1.04mmol) was suspended in anhydrous THF (28mL), cooled to -78°C with a dry ice-acetone bath, and the Grignard reagent solution of vinyl bromide (4.2mL, 1M, 4.2mmol) was added dropwise. , stirred for 3hr. Then the aziridine derivative (1g, 3.48mmol) in anhydrous Et 2 O (11 mL) solution was added dropwise and stirring was continued for 2 hr. After rising to 20°C, stirring was continued for 3 hr. After the reaction was detected by TLC, the reaction was performed with saturated NaHCO 3 The aqueous solution was quenched, the organic phase was separated, and the aqueous phase was extracted with EtOAc (2*30mL), the organic phases were combined, dried, filtered, and concentrated to obtain a crude product, which was then separated by column chromatography to obtain pure O-TBS-2-Boc-amino -4-pentenol (0.90 g, yield 82%). 1 HNMR (CD 3 Cl, ...
Embodiment 2
[0047] 2. Synthesis of 2-Boc-amino-5-hexenoic acid
[0048] The first step: the synthesis of O-TBS-2-Boc-amino-5-hexenol
[0049] CuBrSMe 2 (107mg, 0.522mmol) was suspended in anhydrous THF (28mL), cooled to -78°C with a dry ice-acetone bath, and the Grignard reagent solution of 3-bromopropene (4.2mL, 1M, 4.2mmol) was added dropwise, and When finished, stir for 1.5 hr. Then the aziridine derivative (1g, 3.48mmol) in anhydrous Et 2 O (11 mL) solution was added dropwise and stirring was continued for 1.5 hr. After the reaction was detected by TLC, the reaction was performed with saturated NaHCO 3 The aqueous solution was quenched, the organic phase was separated, and the aqueous phase was extracted with `EtOAc (2*30mL), the organic phases were combined, dried, filtered, and concentrated to obtain a crude product, which was then separated by column chromatography to obtain the product O-TBS-2-Boc-amino -5-Hexenol (1.03 g, yield 90%). 1 HNMR (CDCl 3 , 400MH z ): δ=0.05...
Embodiment 3
[0055] 3. Synthesis of 2-Boc-amino-7-octenoic acid
[0056] Step 1: Synthesis of O-TBS-2-Boc-amino-7-octenol
[0057] CuBrSMe 2 (214mg, 1.04mmol) was suspended in anhydrous THF (35mL), cooled to -78°C with a dry ice-acetone bath, and the Grignard reagent solution of 5-bromopentene (4.2mL, 1M, 4.2mmol) was added dropwise, and After the addition was complete, stir for 1.5 hr. Then the aziridine derivative (1g, 3.48mmol) in anhydrous Et 2 O (11 mL) solution was added dropwise and stirring was continued for 1.5 hr. After the reaction was detected by TLC, the reaction was performed with saturated NaHCO 3 The aqueous solution was quenched, the organic phase was separated, and the aqueous phase was extracted with EtOAc (2*30mL), the organic phases were combined, dried, filtered, and concentrated to obtain a crude product, which was then separated by column chromatography to obtain the product O-TBS-2-Boc-amino- 7-octenol (1.11 g, 89% yield). 1 HNMR (CDCl 3 , 400MH z ): δ=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com