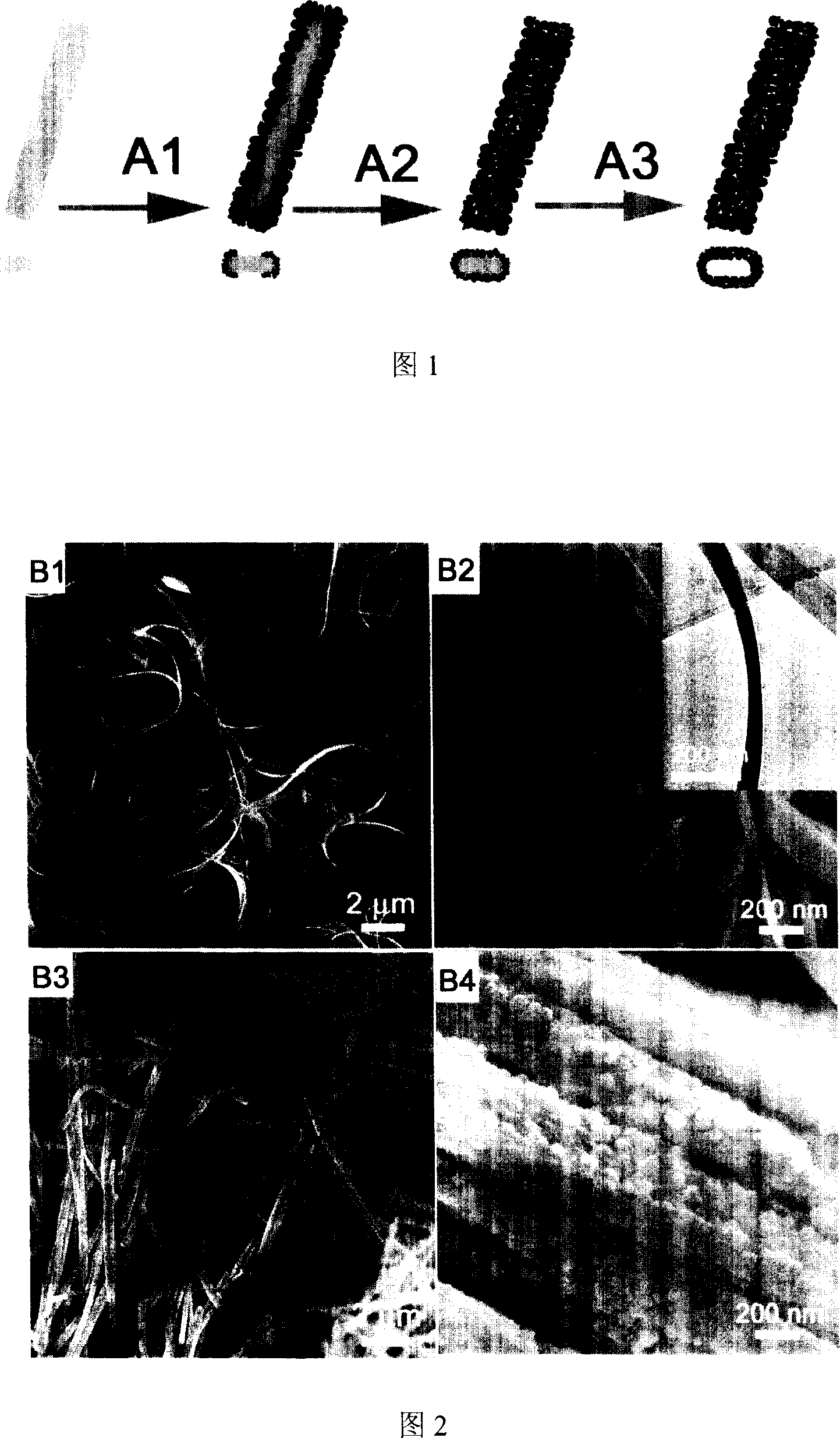
Method for preparing unidimensional TiO2 hollow structured photocatalyst using vanadium oxide nanobelt as template
A hollow structure, photocatalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of complex template method, limited application of template method, hollow structure destruction and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
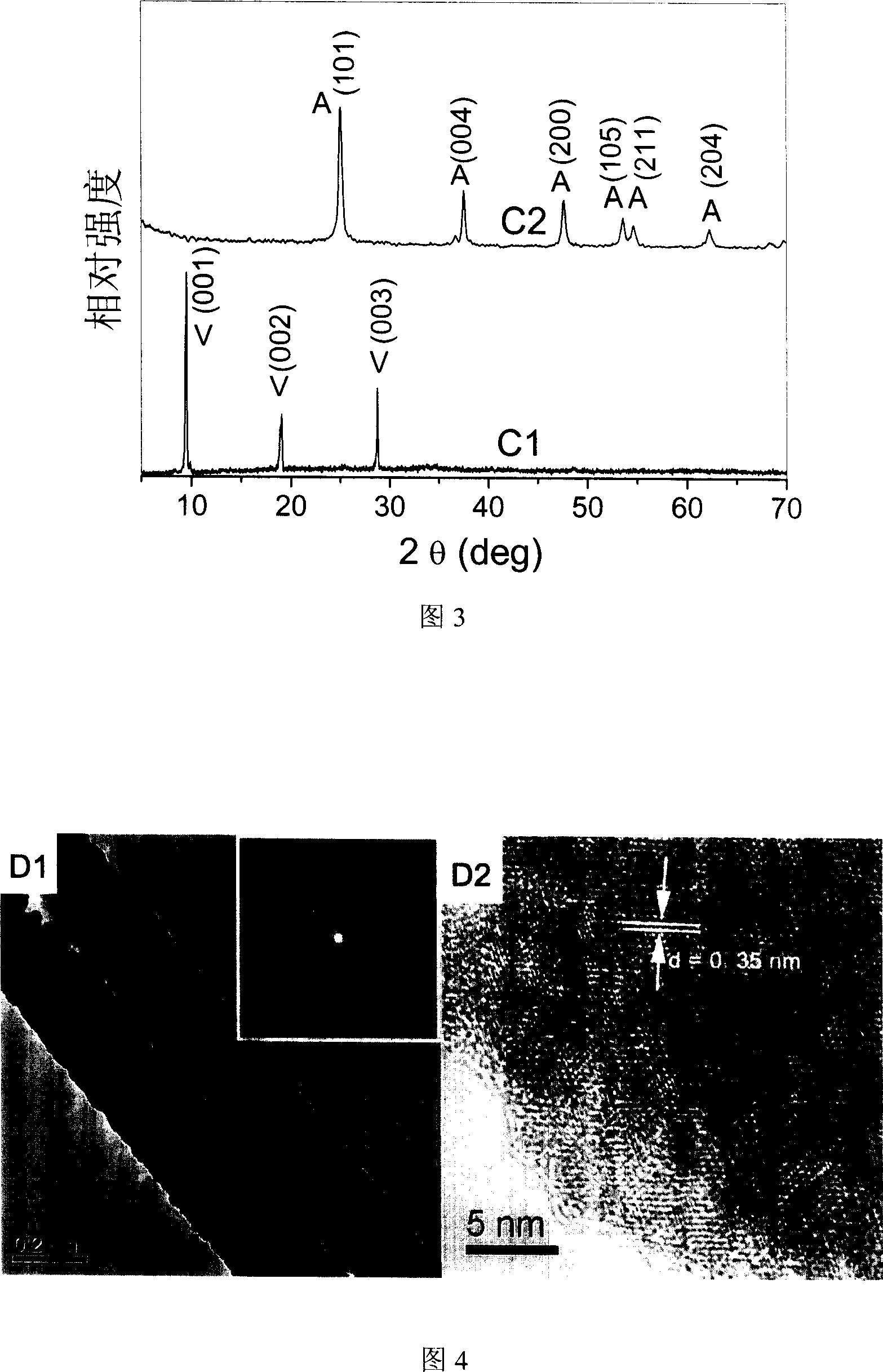
[0026] Preparation of vanadium oxide nanobelt template: Add 0.06 g of ammonium metavanadate and 0.25 g of polyethylene glycol 400 into 30 ml of deionized water and stir evenly, adjust the above solution with 1 mol / L hydrochloric acid and 1 mol / L sodium hydroxide pH to 3. Then the reaction solution was transferred to a 50 ml hydrothermal kettle and kept at 190±2° C. for 24 hours. After the reaction is completed, the precipitate is separated by filtration, washed three times with deionized water and absolute ethanol, and finally vacuum-dried at 60±2° C. for 8 hours to obtain vanadium oxide nanobelts. Figures 2B1 and 2B2 show field emission SEM images of vanadium oxide nanoribbons. It can be seen from the figure that the products prepared by this hydrothermal method are nanoribbon structures with a length of 10-400 micrometers, a width of 100-300 nanometers, and a thickness of about 35 nanometers. Figure 3C1 shows the XRD pattern of the as-prepared nanoribbon structure, indicat...
Embodiment 2
[0031] In order to examine the effect of the concentration of TiF4 on the one-dimensional TiO 2 The influence of hollow structure morphology, except the concentration of titanium tetrafluoride, other reaction conditions such as: the pH value of the reaction solution, the reaction temperature of the solution, the reaction time of the solution, etc. are all the same as in Example 1. The results show that when the concentration of titanium tetrafluoride is higher than 0.1 mol / L, due to the prepared TiO 2 The shell of the hollow structure is very thick, and it is difficult to see the hollow structure with TEM; when the concentration of titanium tetrafluoride is lower than 0.001 mol / L, due to the TiO in the system 2 With less content, the surface of vanadium oxide nanobelts cannot be completely covered, resulting in an uneven hollow structure. At the same time, the decrease in the concentration of titanium tetrafluoride leads to the inability of vanadium oxide nanobelt templates to...
Embodiment 3
[0033] In order to examine the pH value of the reaction solution on the one-dimensional TiO 2 The impact of hollow structure morphology, except the pH value of the reaction solution is different, other reaction conditions such as: the concentration of the reaction solution, the reaction temperature of the solution, the reaction time of the solution, etc. are all the same as in Example 1. The results show that when the pH value of the reaction solution is higher than 6, the solution has an obvious white precipitate, which is deposited on the bottom of the container but not on the surface of the template, and it is difficult to obtain one-dimensional TiO 2 At the same time, the degree of crystallization of titanium dioxide particles decreases due to the increase in the hydrolysis rate of titanium tetrafluoride; when the H of the reaction solution + When the ion is very high (pH+ The presence of ions inhibits the hydrolysis of titanium tetrafluoride, thus requiring longer process...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com