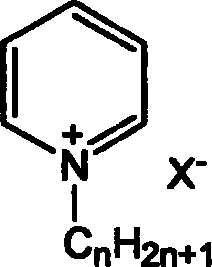
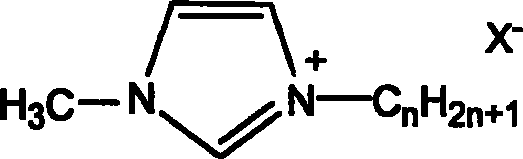
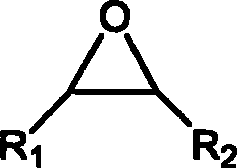
Process for preparing annular carbonate
A technology of cyclic carbonates and epoxy compounds, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problem of low single-component catalytic activity and difficulty in large-scale production , harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] Implementation method: In a 100ml stainless steel autoclave, sequentially add 1.0mmol of tetrabutylammonium bromide, 0.66mol of water, and finally add 0.2mol of propylene oxide (1a), seal the reaction vessel, and charge carbon dioxide with an appropriate amount of pressure. The instrument controls the temperature to rise slowly to 125°C, then controls the carbon dioxide pressure to be 2.0MPa, reacts for 1.0 hour, cools to room temperature, unloads the kettle, absorbs excess carbon dioxide with saturated sodium carbonate solution, and distills the resulting liquid under reduced pressure to obtain the product (2a) And (3a), gas chromatography analysis, (1a) conversion rate 94.5%, product (2a) selectivity 95.4%, yield is 90.2%.
Embodiment 2
[0026] Same as Example 1, the catalyst used is 1.0 mmol of tetrabutylammonium iodide, and the others remain unchanged, the conversion rate of (1a) is 99.5%, the selectivity of product (2a) is 95.5%, and the yield is 95.0%.
Embodiment 3
[0028] Same as in Example 2, the catalyst used is 1.5 mmol of tetraethylammonium iodide, and the others remain unchanged, the conversion rate of (1a) is 100.0%, the selectivity of product (2a) is 95.0%, and the yield is 95.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com