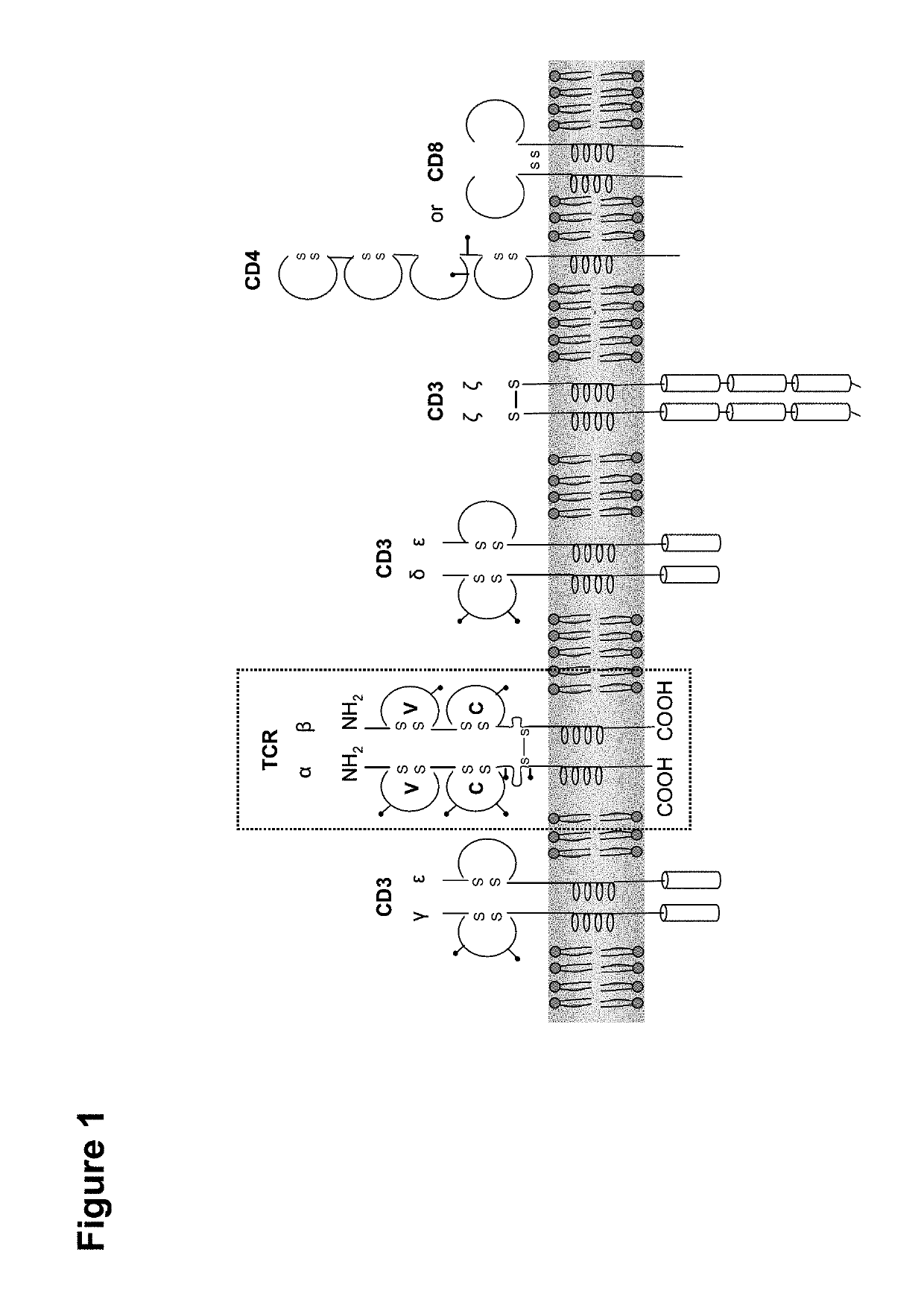
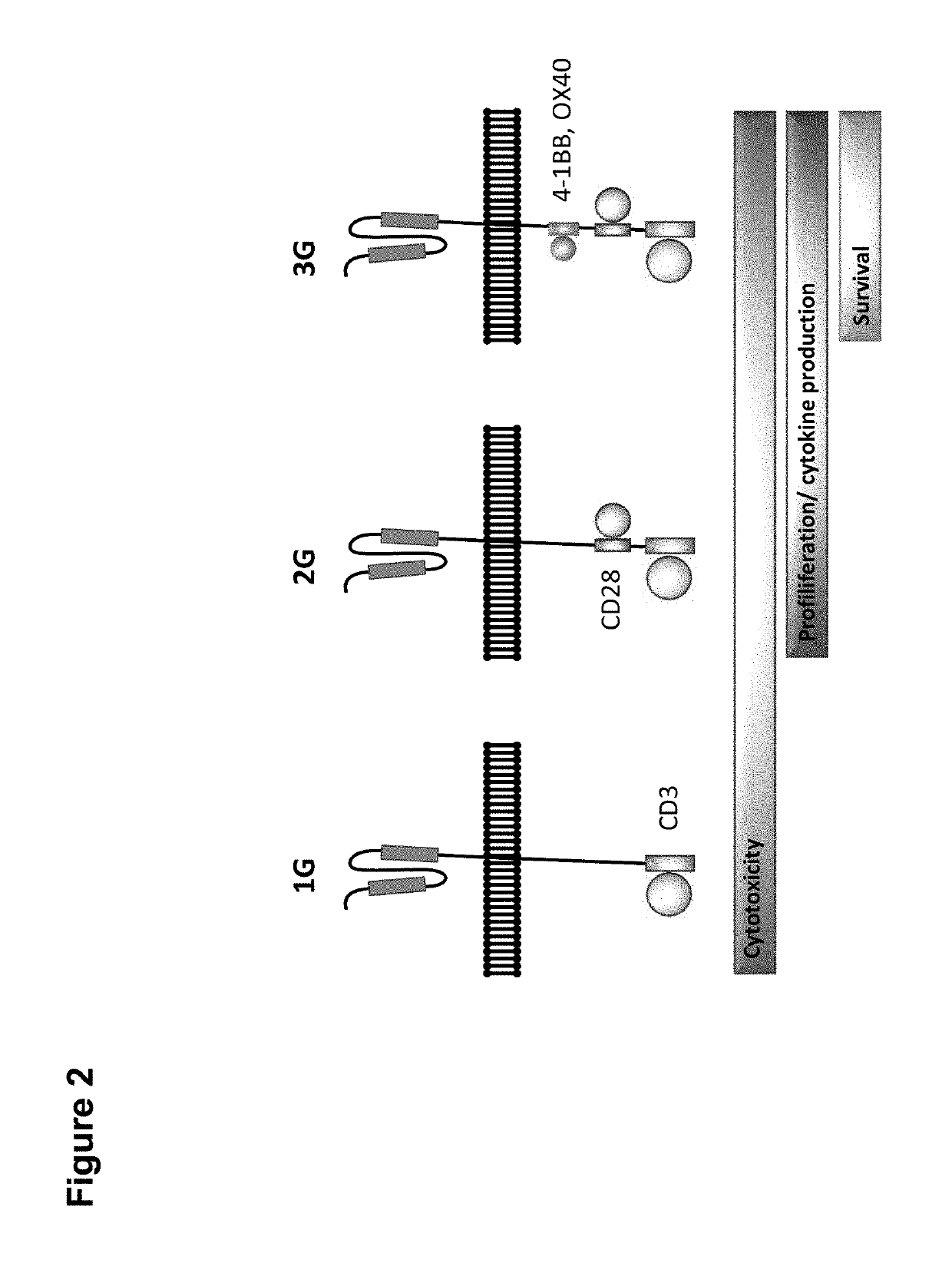
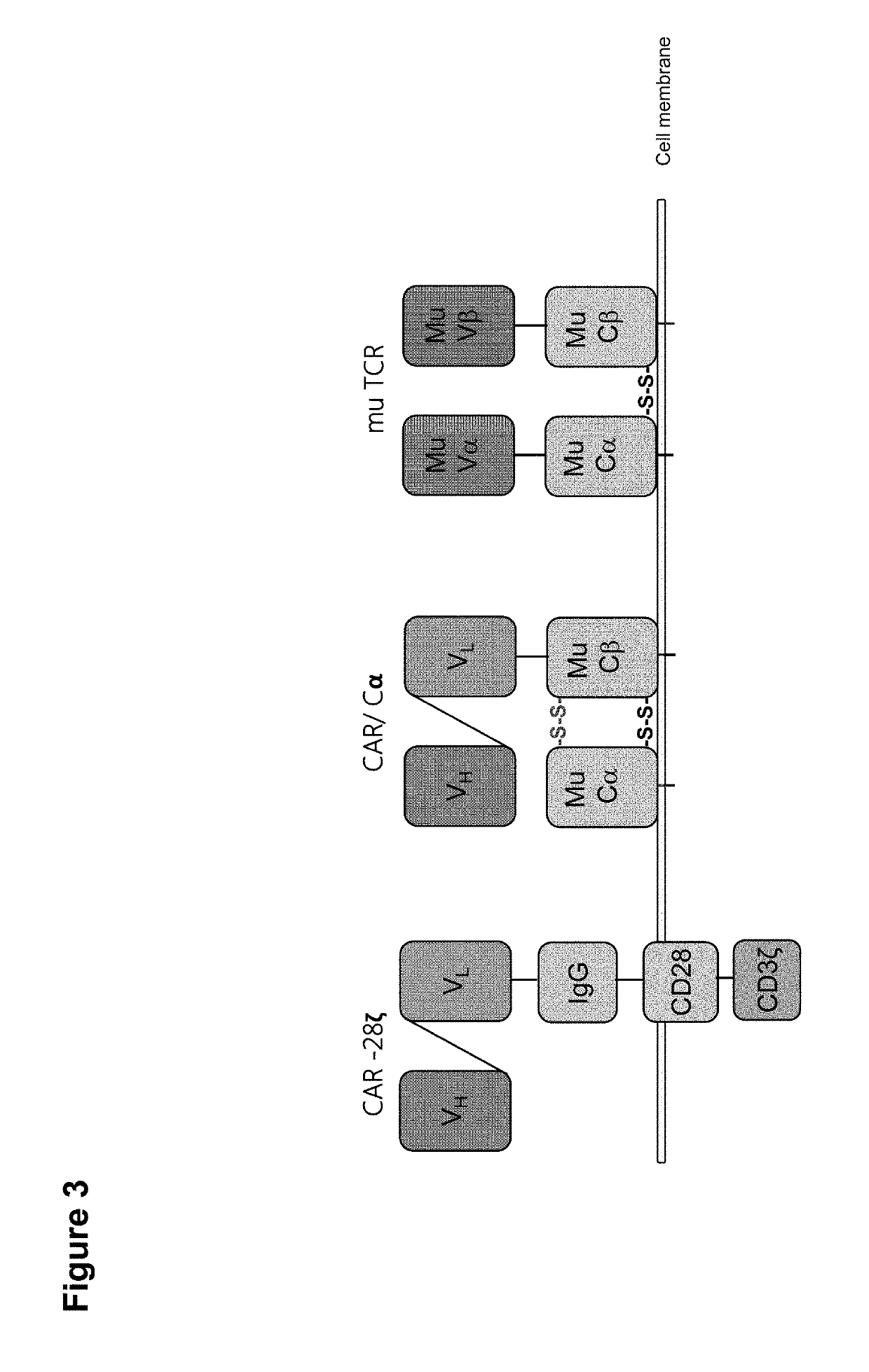
Claudin-6-specific immunoreceptors and T cell epitopes
a technology of immunoreceptors and t cells, applied in the field of claudin-6-specific immunoreceptors and t cell epitopes, can solve the problems of significant morbidity and mortality in immune compromised individuals such as transplant recipients or aid patients, and the inability to systemically correlate immunoreactivity with clinical outcomes, and achieve the effect of minimizing adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
and Methods
[0426]Cell Lines and Reagents
[0427]The human chronic myeloid leukemia cell line K562 (Lozzio, C. B. & Lozzio, B. B (1975), Blood 45, 321-334) was cultured under standard conditions. K562 cells stably transfected with HLA-A*0201 (Britten, C. M. et al. (2002), J. Immunol. Methods 259, 95-110) (referred to e.g. as K562-A*0201) were used for validation assays. The primary human newborn foreskin fibroblast cell line CCD-1079Sk (ATCC No. CRL-2097) was cultured according to the manufacturers' instructions.
[0428]The human CLDN6 expressing ovarian carcinoma cell line OV-90-SC12 was used for in vivo validation of the CLDN6-CAR.
[0429]The culture medium for PA-1-SC12_A0201_luc_gfp_F7 is composed of 86% RPMI 1640+ Glutamax (Co. Gibco, Cat-No. 61870), 10% FCS (Co. Biochrome, Cat-No. S0615), 1% Sodium Pyruvate (100 mM) (Co. Gibco, Cat-No. 11360), 1% MEM Non-Essential Amino Acids Solution (100×) (Co. Gibco, Cat-No. 11140), 2% Sodium Bicarbonate 7, 5% solution (Co. Gibco, Cat-No. 25080).
[...
example 2
of High-Affinity HLA-A*02-Restricted Murine TCRs Specific for Claudin-6
[0475]We validated the immunogenic potential of CLDN6 in A2 / DR1 mice by repetitive intranodal immunization with CLDN6 encoding IVT-RNA and used spleen cells of these mice for isolation of CLDN6-specific T cells and subsequent cloning of the corresponding TCR genes (FIG. 5). Spleen cells of immunized mice were analyzed for the successful induction of CLDN6-specific T cells and their reactivity against predicted HLA-A*02 binding CLDN6 peptides ex-vivo by IFNγ-ELISPOT assay (FIG. 6).
[0476]Significant frequencies of CLDN6-specific T cells could be induced In all three mice by RNA immunization, whereas T cell reactivity was focused on two CLDN6 peptides predicted, that were with the best HLA-A*02 binding score (Cl6-A2-1 and Cl6-A2-2).
[0477]For isolation of CLDN6-specific T cells, spleen cells of immunized mice were restimulated in-vitro and sorted by flow cytometry based on the activation-induced upregulation of CD137...
example 3
ve Testing of Murine TCRs Specific for CLDN6 91-99
[0480]In total six murine TCRs were identified that all recognize the HLA-A*02-restricted epitope CLDN6-91-99. In order to confirm that this epitope is naturally processed and presented by endogenously CLDN6 expressing tumor cell lines and to evaluate the potential of the identified murine TCRs to mediate killing of such cells a luciferase-based cytotoxicity assay was performed. Human preactivated CD8+ T cells were transfected with TCR RNA and surface expression was analyzed by flow cytometry (FIG. 9). All murine TCRs were expressed on a high percentage of human CD8+ T cells after RNA transfer as indicated by staining with an fluorochrome-conjugated antibody specific for the constant domain of the murine TCR-β chain. TCR-transfected T cells were subjected to luciferase-based cytotoxicity assay together with the CLDN6-expressing tumor cell lines PA1 (teratoma) and NIH-Ovcar3 (ovarian carcinoma). The CLND6-negative breast cancer cell l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dissociation constant | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com