Method and apparatus for producing nitrogen from air by cryogenic distillation
a technology of cryogenic distillation and air, which is applied in the direction of lighting and heating apparatus, refrigeration and liquid storage, solidification, etc., can solve the problem that simple two-column systems do not necessarily have lower unit energy requirements, and achieve the effect of low energy requirements and efficient raising
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
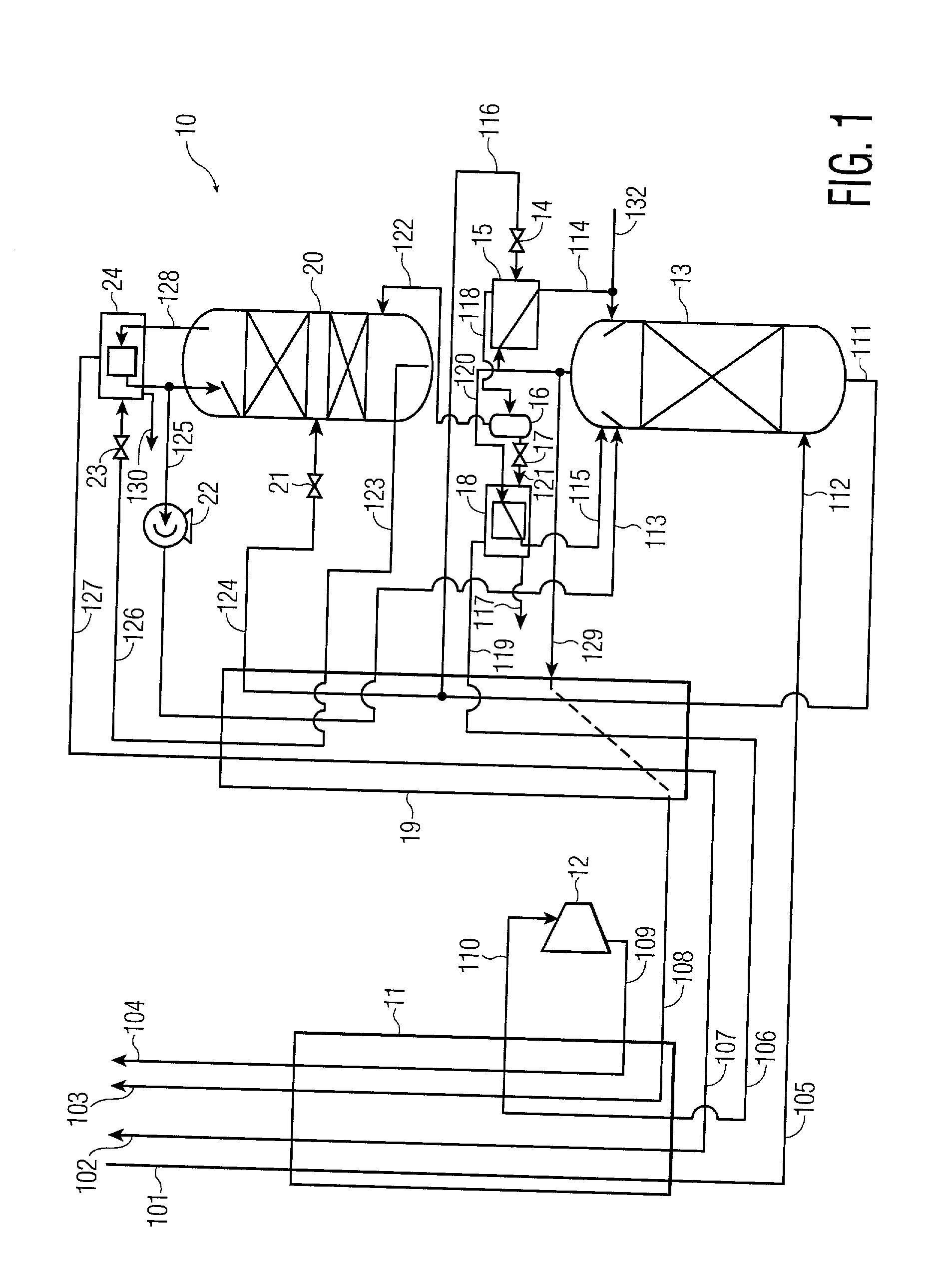
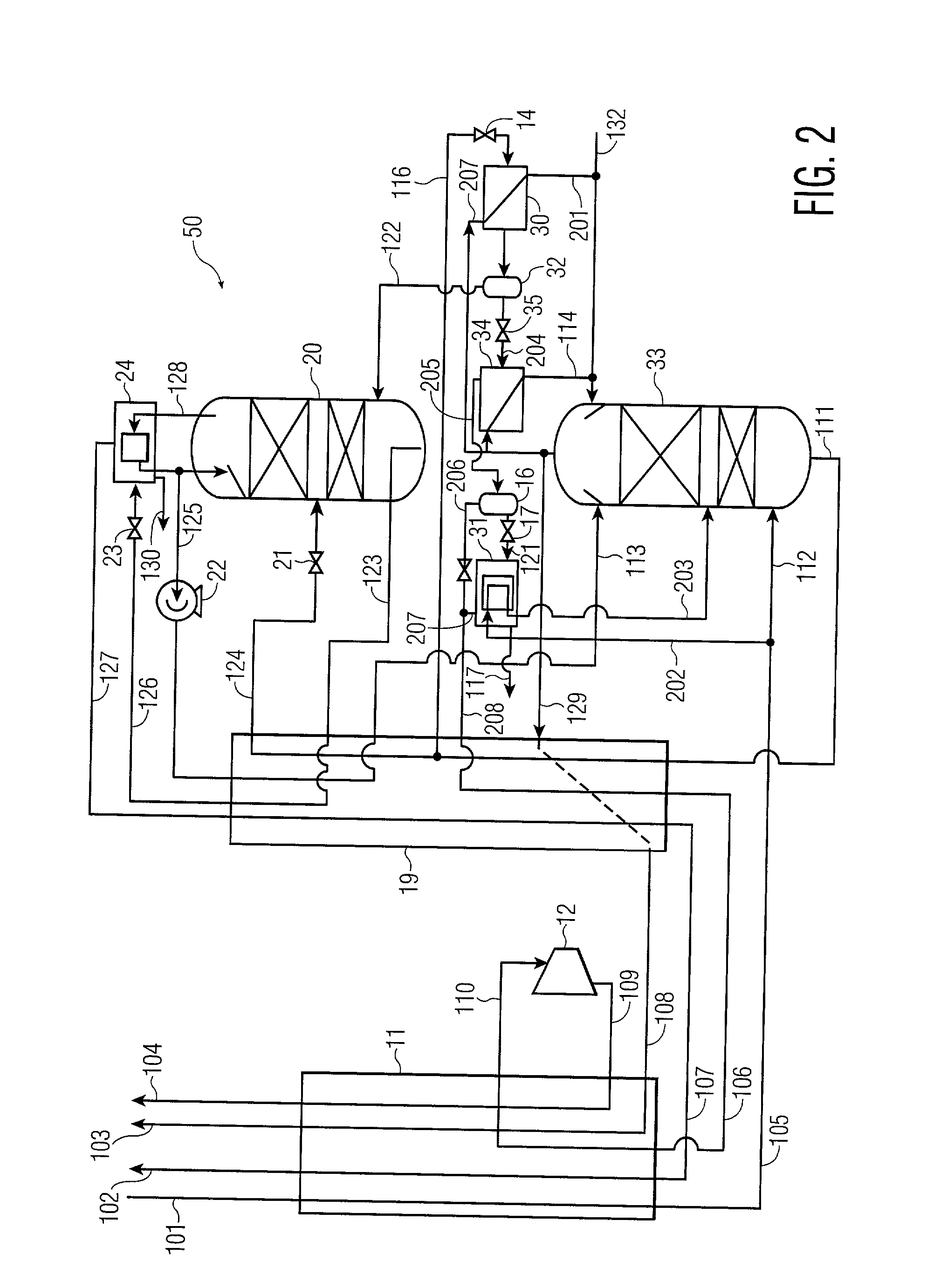
[0026] A process for the recovery of substantially pure nitrogen at a rate of 2687 Nm3 / hr at a pressure of 4.9 atma is conducted in accordance with FIG. 1. Nm3 / hr refers to the flow rate of a substance measured as a gas at 0 C. and 1 atma. C. refers to temperature in degrees Celsius; atma refers to pressure in absolute atmospheres. K refers to temperature in degrees Kelvin.
[0027] A feed air flow of 4632 Nm3 / hr was compressed to a pressure of 5.2 atma, aftercooled to about ambient temperature, its water condensate removed, and passed to an adsorption unit for removal of water and carbon dioxide, and possibly other contaminants. The purified air 101 was passed to main heat exchanger 11 where it was cooled to approximately its dew point, producing a small amount of liquid. Air 105 entered the bottom of high pressure column 13 at 98.6 K and 5.05 atma. The high pressure column is internally made up of structured packing for mass transfer.
[0028] Gaseous nitrogen at a 94.1 K and 5.0 atma e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com