Method for brightening mechanical pulps
a mechanical pulp and brightening technology, applied in pulping with acid salts/anhydrides, papermaking, paper pulping, etc., can solve the problems of less effective technique at higher ph values, the presence of transition metal ions in paper pulp is known to be detrimental to the brightening process, etc., to reduce the bleaching efficiency, increase the brightness reversion, and reduce the brightness level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
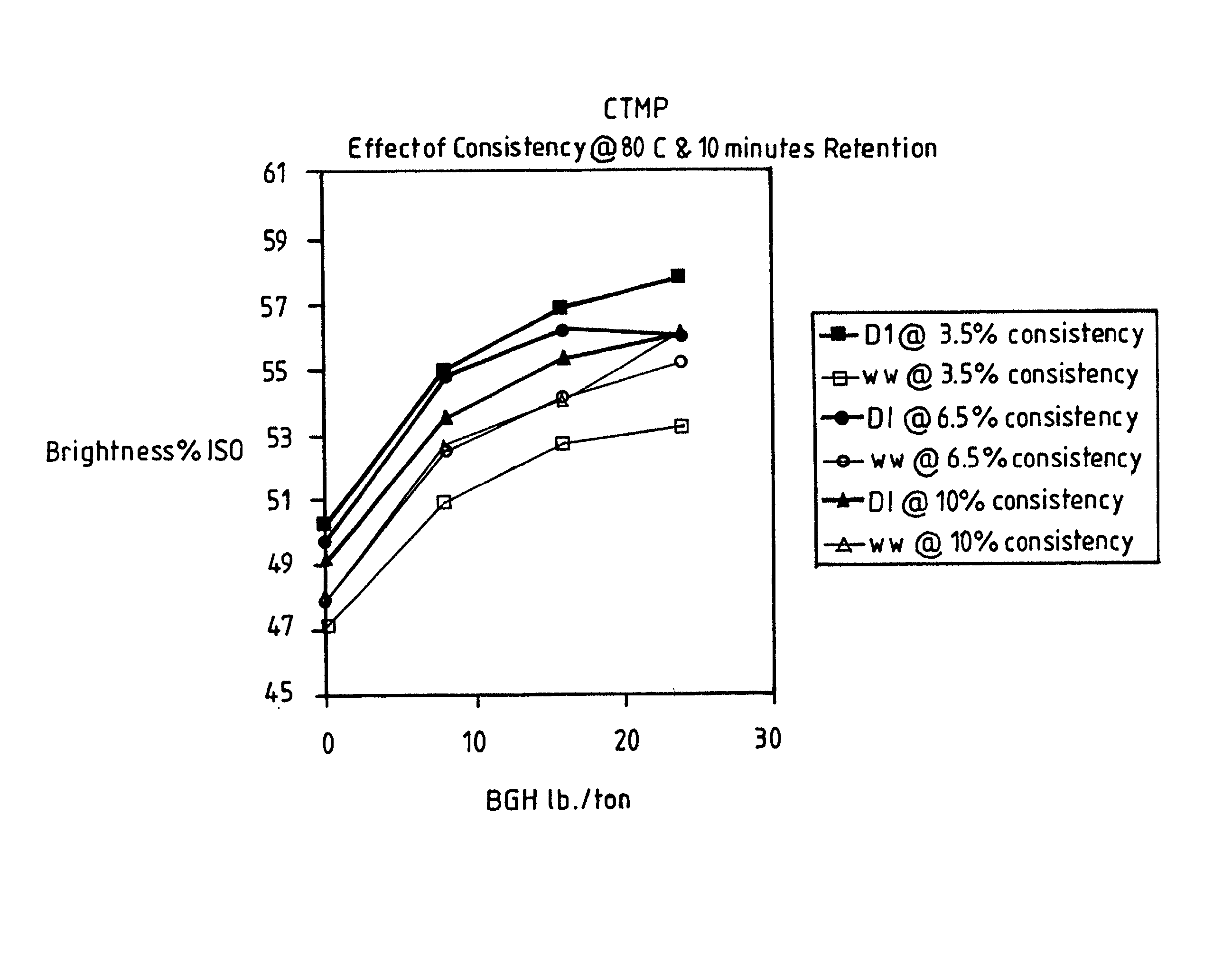
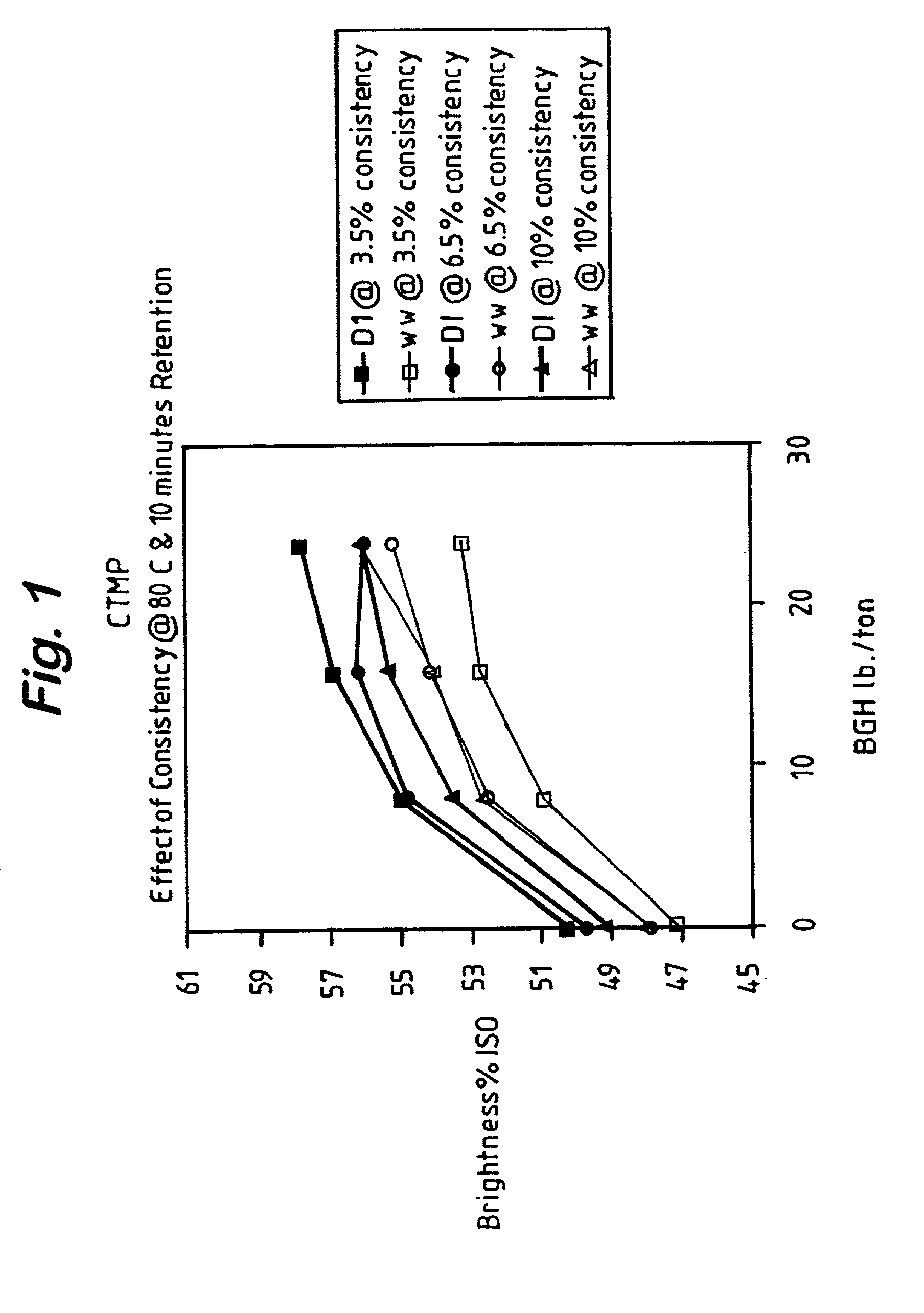
Effect of Process Conditions on BGH Brightening
[0017] Pulp and white water used in this study were obtained from a North American mill. The pulp was a chemothermomechanical pulp (cTMP), which was collected after the secondary refiners and prior to the latency chest. The Precipitated Calcium Carbonate (PCC) containing white water (WW) was collected just prior to dilution at the latency chest. Studies were conducted to determine the effect on BGH bleached pulp brightness levels of the following four process variables: retention time, bleaching temperature, and bleaching consistency using either PCC-containing white water (WW) or deionized (DI) water for dilution of the pulp slurry. The hydrosulfite dosage (hydro) was 0-24 lbs. / ton BGH at a pH of 10. The raw data for these experiments can be seen in Table 1. Temperatures are in .degree. C. (Temp.), retention times are in minutes, consistency ("Consist.") in weight % of pulp in the pulp slurry, deionized water had a pH of 6.5 and PCC-co...
example 2
Fines (Solids) Removal and Reuse of Low-Solids White Water
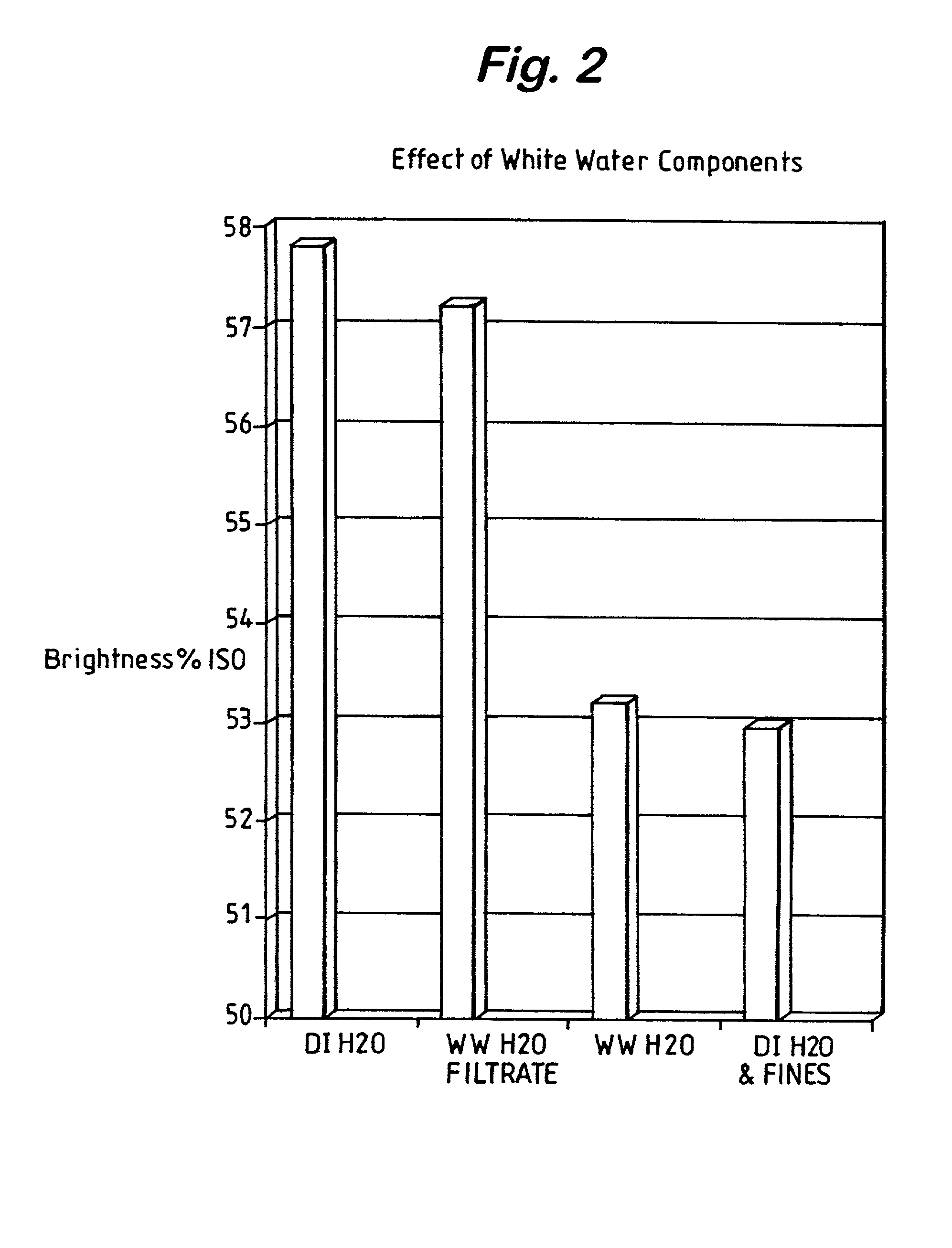
[0022] FIG. 2 compares the BGH-bleached pulp brightness of pulp diluted with DI water, PCC-containing white water, the filtrate of PCC-containing white water, and fines that were removed by filtration of PCC-containing white water and re-suspended in DI water. The results show that the removal of solids and reuse of low solids white water for bleaching purposes minimizes the adverse effects of BGH brightening under alkaline or neutral conditions. The results also show that it is the fines portion, which consists of actual pulp fines, undissolved solids, and transition metals in the white water that is responsible for most of the brightness loss. Based on these results, further testing of the fines portion of PCC containing white water was undertaken and is summarized in the following examples.
[0023] Results of transition metal analysis of the pulp, the white water filtrate, and the fines are shown in Table 5.
5TABLE 5 Metals C...
example 3
Fines Treatment and Re-use
[0025] To reduce the transition metal concentrations, fines were treated by the Qy process. The fines first were treated with 0.1% BGH and then with 0.5% diethylenetriaminepentaacetic acid (DTPA). Experiments were conducted at a pH of 5.5, a consistency of 3.0%, and a temperature of 50.degree. C. for 30 minutes. The Qy treatment is believed to be more effective than the Q treatment, i.e., use of only chelant, because reduction of transition metal valence state by BGH renders the transition metal ions more amenable to chelation. The Qy treatment allows higher brightness levels when using hydrogen peroxide as a brightening agent. Table 6 shows the results from Q and Qy treatments on the fines portion of PCC-containing white water.
6TABLE 6 Metal Levels After Q or Qy Treatment of Fines From PCC-Containing White Water (levels in ppm, unless otherwise indicated) Al Ca (%) Cu Fe Mg Mn Q 807 8.23 4.3 668 336 76.1 Qy 726 6.84 3.3 638 312 61.2 Control 821 9.99 3.4 66...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com