Optical determination of glucose utilizing boronic acid adducts
a technology of boronic acid and glucose, applied in the field of optical determination of glucose utilizing boronic acid adducts, can solve the problems of inability to develop and commercialize in vivo monitoring systems, all prior art sensors are deficient in one or more aspects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation b
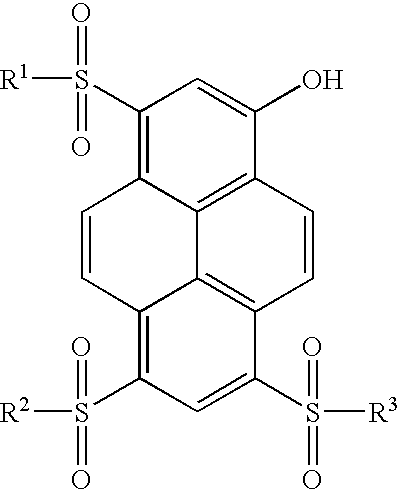
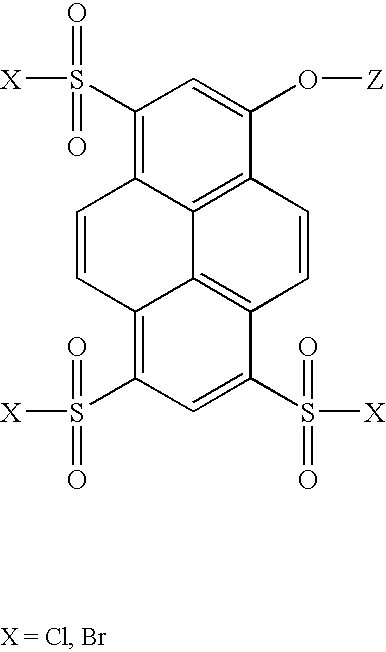
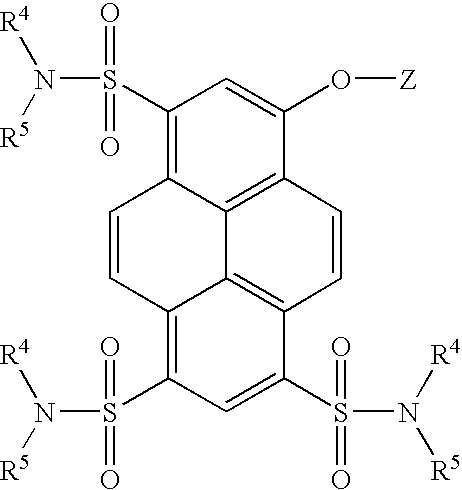
Synthesis of 8-acetoxy-pyrene-1,3,6-trisulfonyl Chloride
[0237] Trisodium-8-acetoxy-pyrene-1,3,6-trisulfonate (acetoxy-HPTS, 11.33 g, 20 mmol) was suspended in 30 mL of thionyl chloride to which 5 drops of dimethylformamide was added. The suspension was refluxed for 3 hr., during which time it became a brown solution. The solution was then cooled to 25.degree. C. under an argon atmosphere. Thionyl chloride was then distilled off under vacuum (2 Torr) leaving a yellow residue. The yellow residue was transferred to three separate centrifuge tubes along with 60 mL of dichloromethane. The suspensions were then centrifuged and the supernatant solutions transferred to a dry round bottom flask. The residue remaining in the centrifuge tubes was washed an additional four times each with 10 mL portions of dichloromethane. The supernatant solutions were combined and left overnight under an argon atmosphere and some precipitation was observed.
[0238] The dichloromethane solution was added to 250 ...
preparation d
Synthesis of N-benzyl-4-ethenyl-4,7-phenanthrolinium Chloride (4,7-Phen SV)
[0240] A flame dried, side armed 100-mL round bottom flask, equipped with a magnetic stirring bar, was cooled under argon and charged with 4,7-phenanthroline (2.14 g, 11.86 mmols). The flask was equipped with a reflux condenser attached to an argon (g) line and charged with 4-(chloromethyl)styrene (0.905 g, 0.836 mL, 5.93 mmols) and anhydrous CH.sub.3CN (20 mL) through the side arm. The solution was heated to reflux under argon (g) for 17 h, then cooled to room temperature and precipitated with diethyl ether (30 mL). The suspension was allowed to settle and the supernatant removed via cannula. The remaining residue along with 15 mL of solvent was cannulated into a centrifuge tube, triturated with acetone (20 mL), and centrifuged (process repeated 4 times). The brownish / pink solid was triturated with diethyl ether (3.times.20 mL) and dried under reduced pressure. Yield: 0.376 g, 1.13 mmols (19%). .sup.1H NMR (...
example 1
Synthesis of 4,4'-N,N'-bis-(benzyl-3-boronic Acid) Dipyridinium Dibromide
[0242] An oven-dried, 50-mL centrifuge tube was cooled under argon, fitted with a magnetic stirring bar, and charged with 4,4'-bipyridyl (0.469 g, 3 mmols). The tube was sealed with a septum and charged with CH.sub.3OH (7 mL). The homogenous solution was stirred at room temperature while freshly prepared dimethyl-(3-bromomethyl)-benzeneboronate (1.82 g, 7.5 mmols) was added via syringe. After stirring the solution for 15 hours, the reaction vessel was centrifuged (4 min at 3200 RPM) and the CH.sub.3OH cannulated to a separate flask. The remaining yellow solid was triturated with acetone:water (24:1, V / V, 25 mL), stirred vigorously on a vortex mixer and centrifuged. The acetone solution was removed by cannula and the trituration process repeated two more times. The solid was then triturated with diethyl ether using the same process. The pale yellow solid, in the centrifuge tube, was then dried on the high vacuum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com