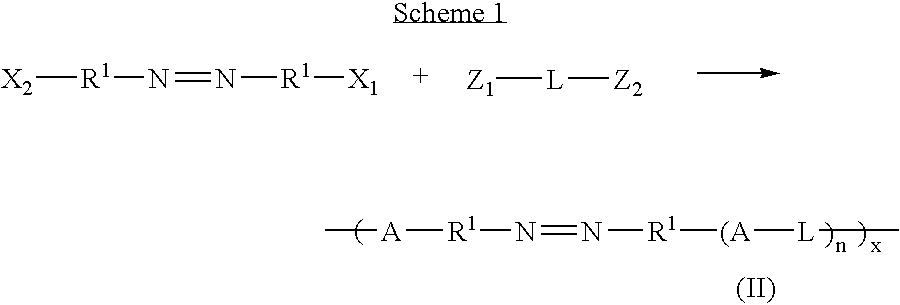
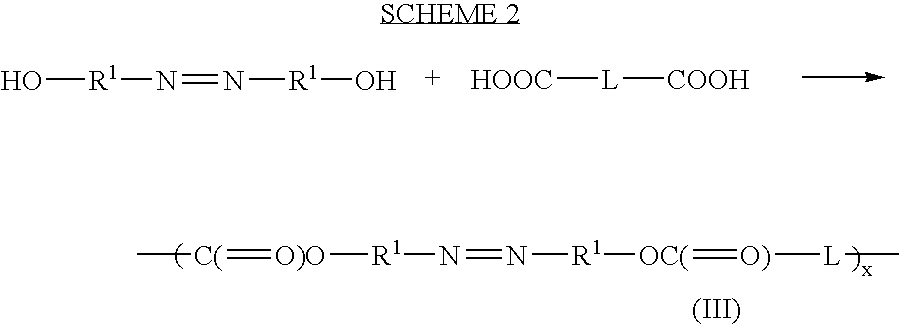
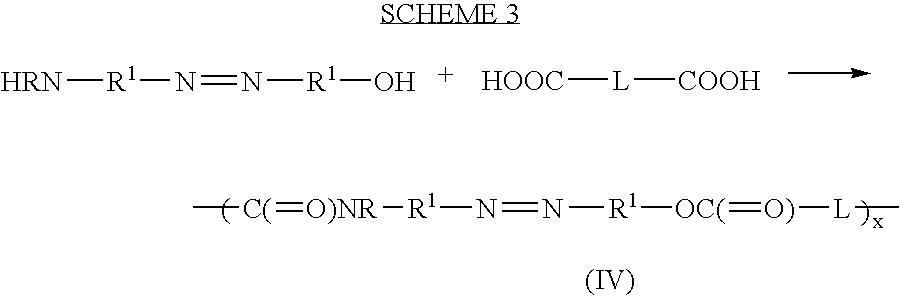
Therapeutic AZO-compounds for drug delivery
a technology of azocompounds and drugs, applied in the direction of medical preparations, pharmaceutical non-active ingredients, etc., can solve the problems of rash, nausea and vomiting, carrier molecule of this component is associated with several side effects,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0108] In Example 2, Scheme 6 illustrates the synthesis of a poly(azo-anhydride) compound linking 4-ASA. The 4-aminosalicylic acid is converted to its methyl ester with sulfuric acid in methanol. Methyl-4-aminosalicylate is dimerized via azoxy linkage with hydrogen peroxide in acetic acid. The azoxy compound is reduced to the hydrazo compound with zinc dust in acetic acid. The hydrazo compound is oxidized to the azo compound with sodium perborate in acetic acid. The methyl esters are cleaved with alkali to give the prepolymer azo diacid. The diacid is converted to the activated monomer by refluxing in an excess of acetic anhydride. The monomer is then converted to the polyazo compound. 6
[0109] Activity
[0110] The ability of a polymer of the invention to produce a given therapeutic effect can be determined using in vitro and in vivo pharmacological models which are well known to the art.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| average molecular weights | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com