Methods and compositions for poly-beta-1-4-N-acetylglucosamine cell therapy system
a cell therapy system and polybeta-1-4-n-acetylglucosamine technology, applied in the field of polysaccharides, can solve the problems of slow growth of chitin-based industries, unpredictability of chitin-based properties, and confusion between “chitin” and “chitosan” in fact, and achieve the effect of easy production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
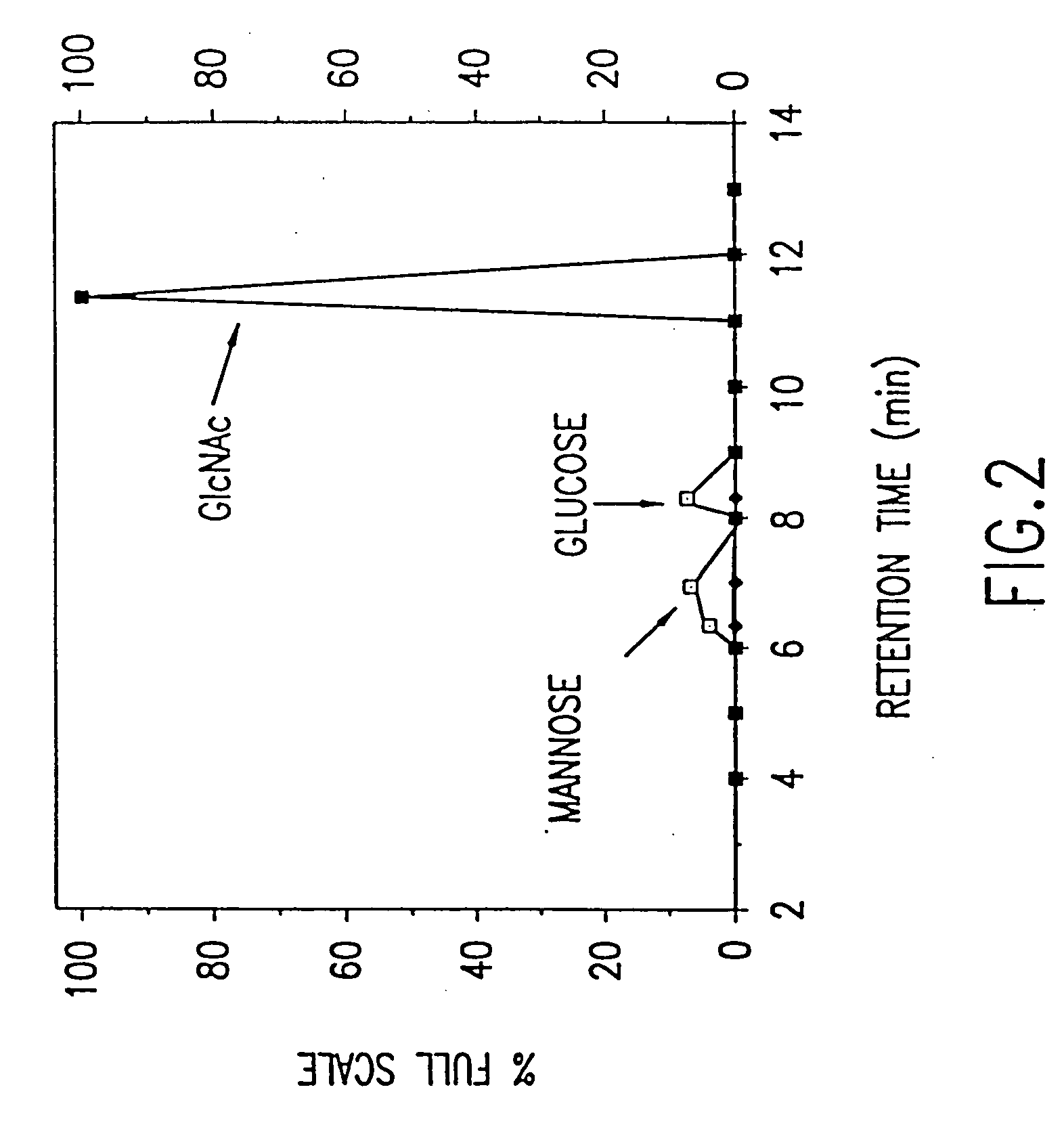
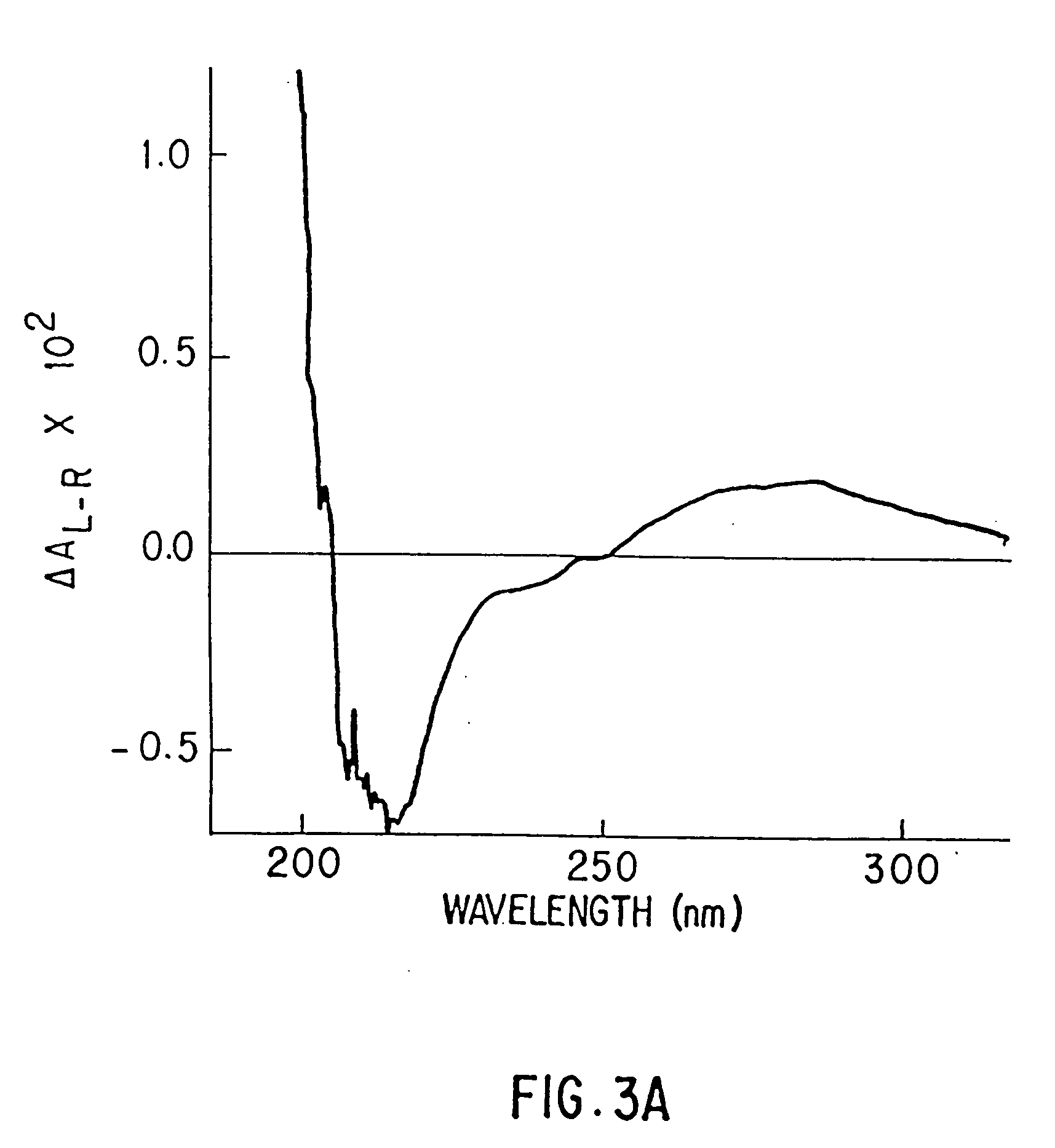
Presented below, is, first, a description of physical characteristics of the purified p-GlcNAc species of the invention, of the p-GlcNAc derivatives, and of their reformulations. Next, methods are described for the purification of the p-GlcNAc species of the invention from microalgae, preferably diatom, starting sources. Third, derivatives and reformulations of the p-GlcNAc, and methods for the production of such derivatives and reformulations are presented. Finally, uses are presented for the p-GlcNAc, p-GlcNAc derivatives and / or p-GlcNAc reformulations of the invention.
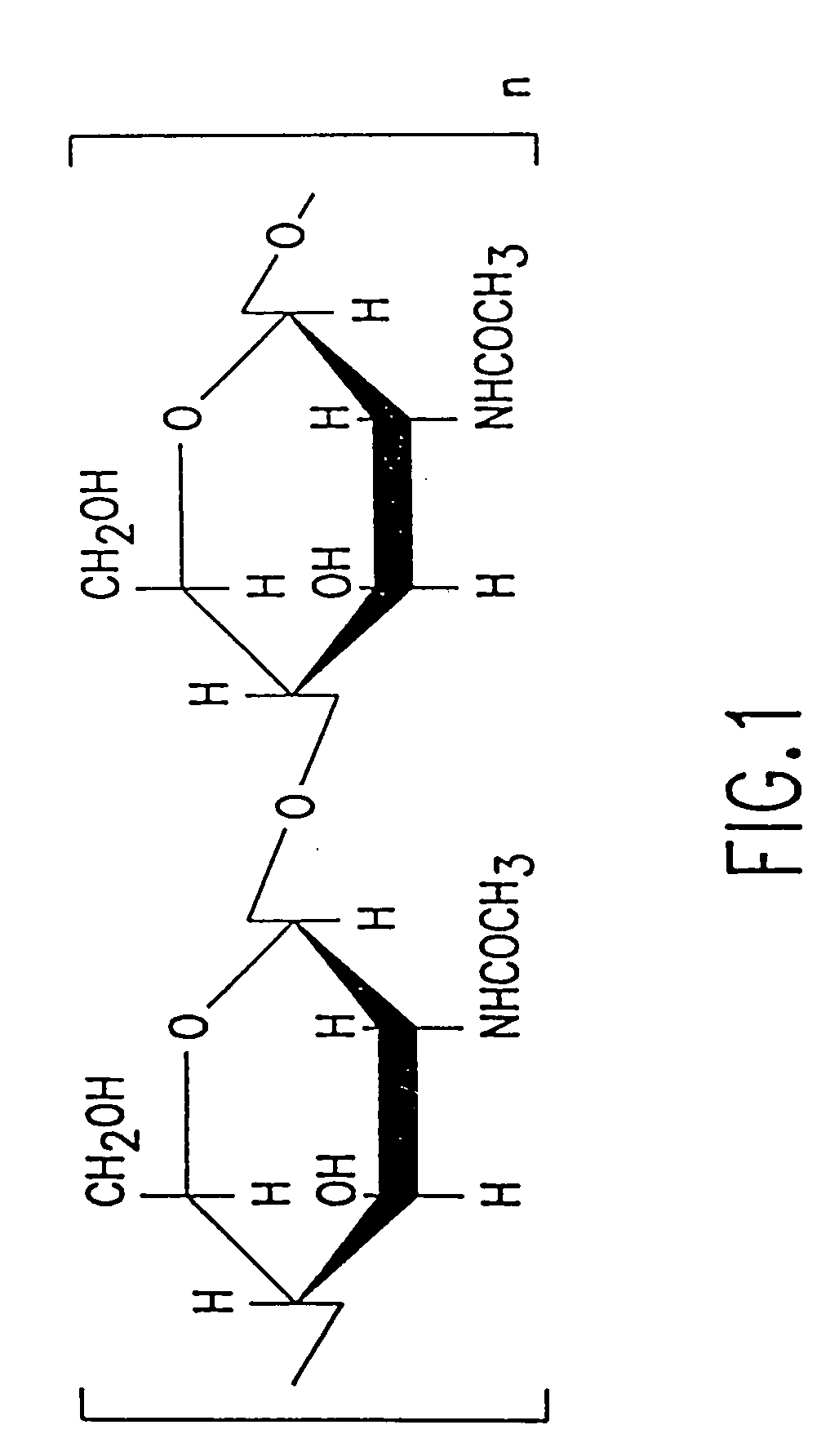
5.1. p-GlcNAc
The p-GlcNAc polysaccharide species of the invention is a polymer of high molecular weight ranging from a weight average of about 800,000 daltons to about 30 million daltons, based upon gel permeation chromatography measurements. Such a molecular weight range represents a p-GlcNAc species having about 4,000 to about 150,000 N-acetylglucosamine monosaccharides attached in a β-1→4 configuration, with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com