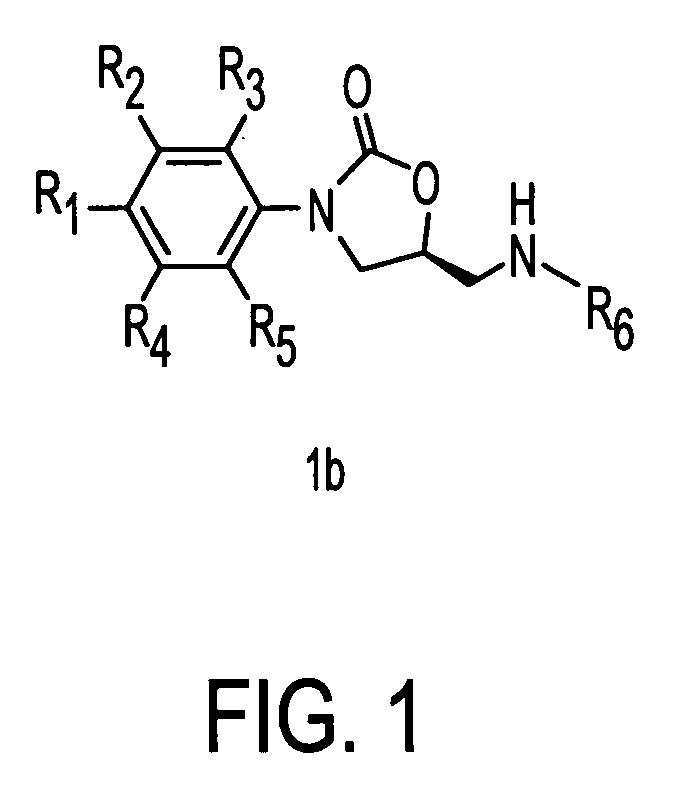
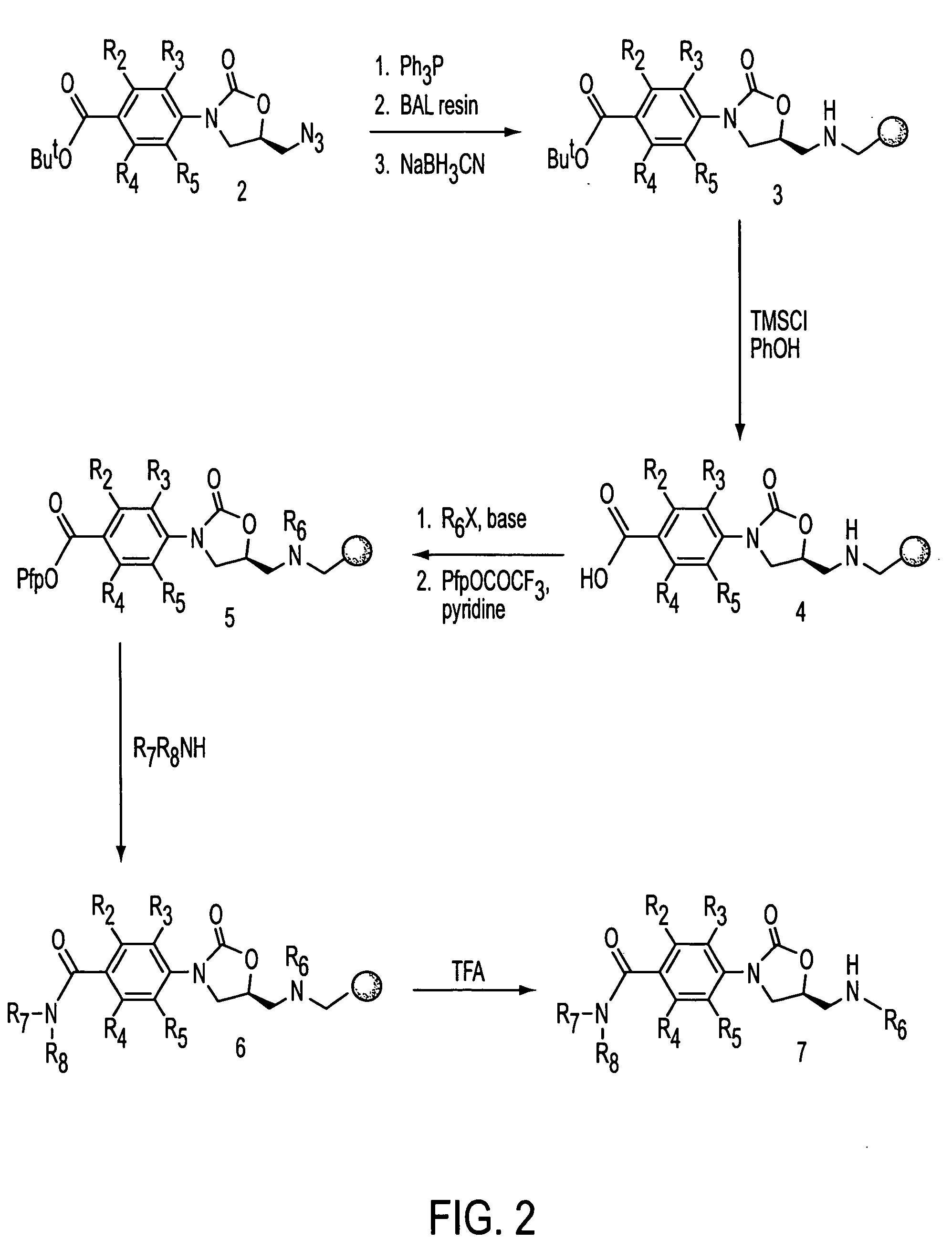
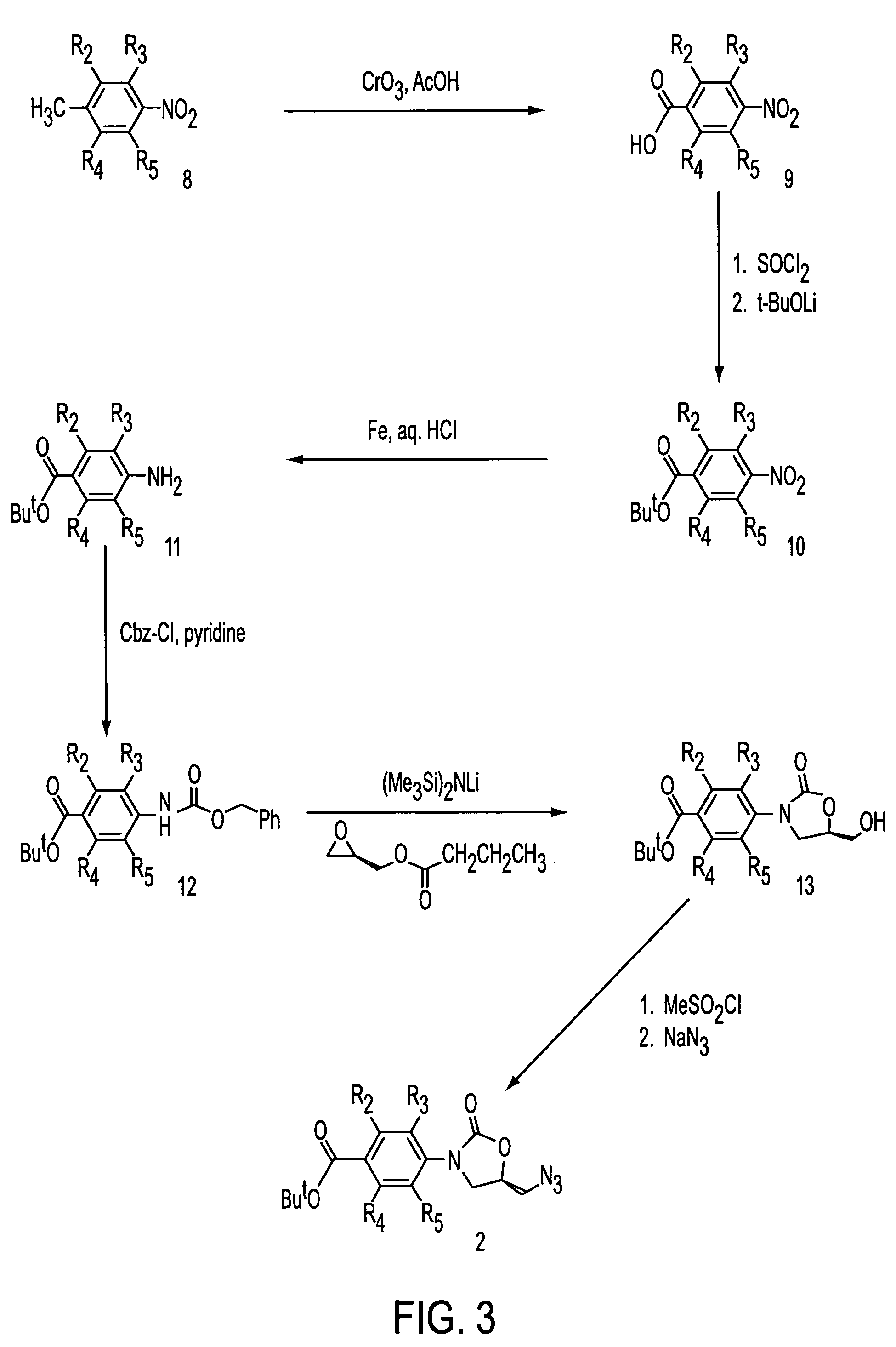
Oxazolidinone combinatorial libraries, compositions and methods of preparation
a technology of combinatorial libraries and oxazolidinones, applied in the field of oxazolidinones, can solve the problems of inability to provide the number of compounds required for a high-throughput biological screen, and provide a limited number of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Abbreviations: ACN, acetonitrile; CDI, carbonyldiimidazole; DIEA, diethylisopropylamine; DCM, dichloromethane; DIC, diisopropyldiimide; DMF, dimethylformamide; HATU, O-(7-azabenzotriazol-1-yl)- 1,1,3,3-bis(tetramethylene)-uronium hexafluorophosphate; NMM, N-methyl morpholine; mCPBA, m-chloro-peroxybenzoic acid; TFA, trifluoroacetic acid; THF, tetrahydrofuran; TMOF, trimethylorthoformate.
General. Reagents were obtained from Aldrich (St. Louis, Mo.), Sigma (St. Louis, Mo.), Bachem Biosciences, Rapp Polymere, Perseptive, and Novabiochem, and used without further purification. The resin Tentagel S NTi was purchased from Rapp Polymere. Concentration of solutions after workup was performed by reduced pressure rotary evaporation, or using the Savant's SpeedVac instrument. Reactions with moisture-sensitive reagents were performed under nitrogen atmosphere.
Mass-spectra were obtained using ESI technique. HTLC analysis and purification were performed using Beckman System Gold R®; detectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com