Catalysts for water gas shift reaction, method for removing carbon monoxide in hydrogen gas and fuel cell generation system
a technology of water gas shift and catalyst, which is applied in the direction of physical/chemical process catalysts, combustible gas catalytic treatment, bulk chemical production, etc., can solve the problem of difficulty in applying cu—zn based catalyst to the fuel cell generation system, and achieve the effect of reducing the concentration of carbon monoxide in the gas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
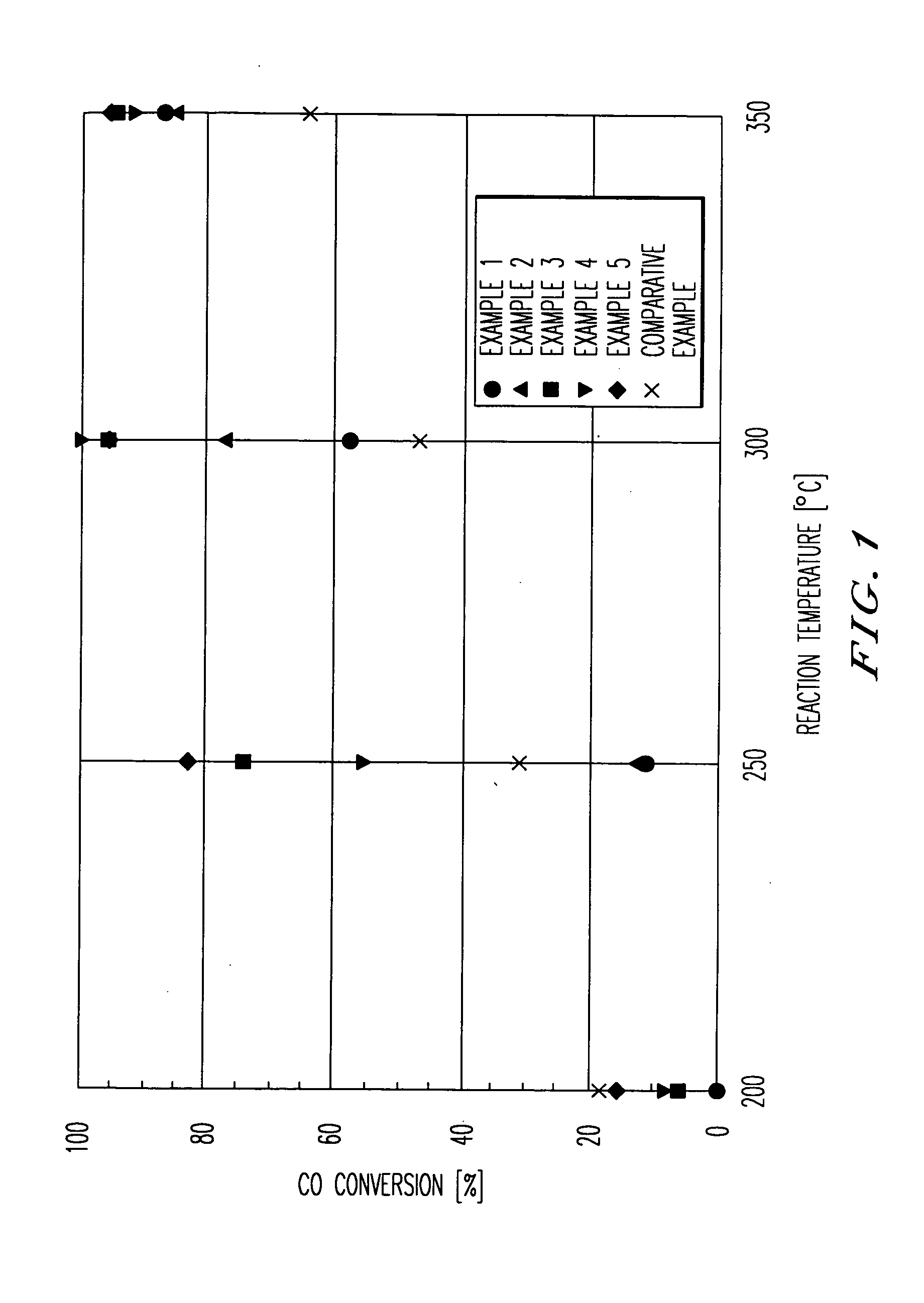
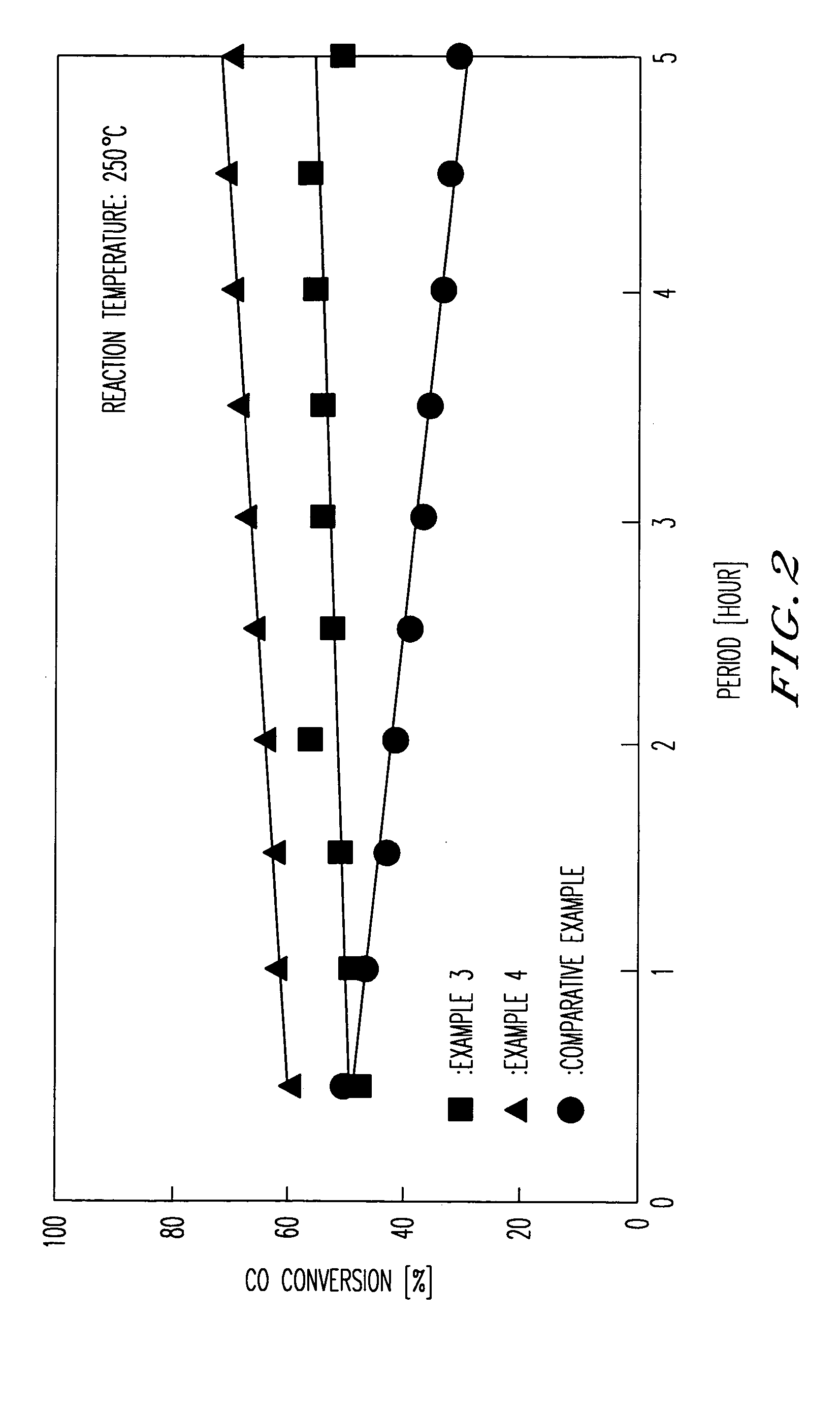
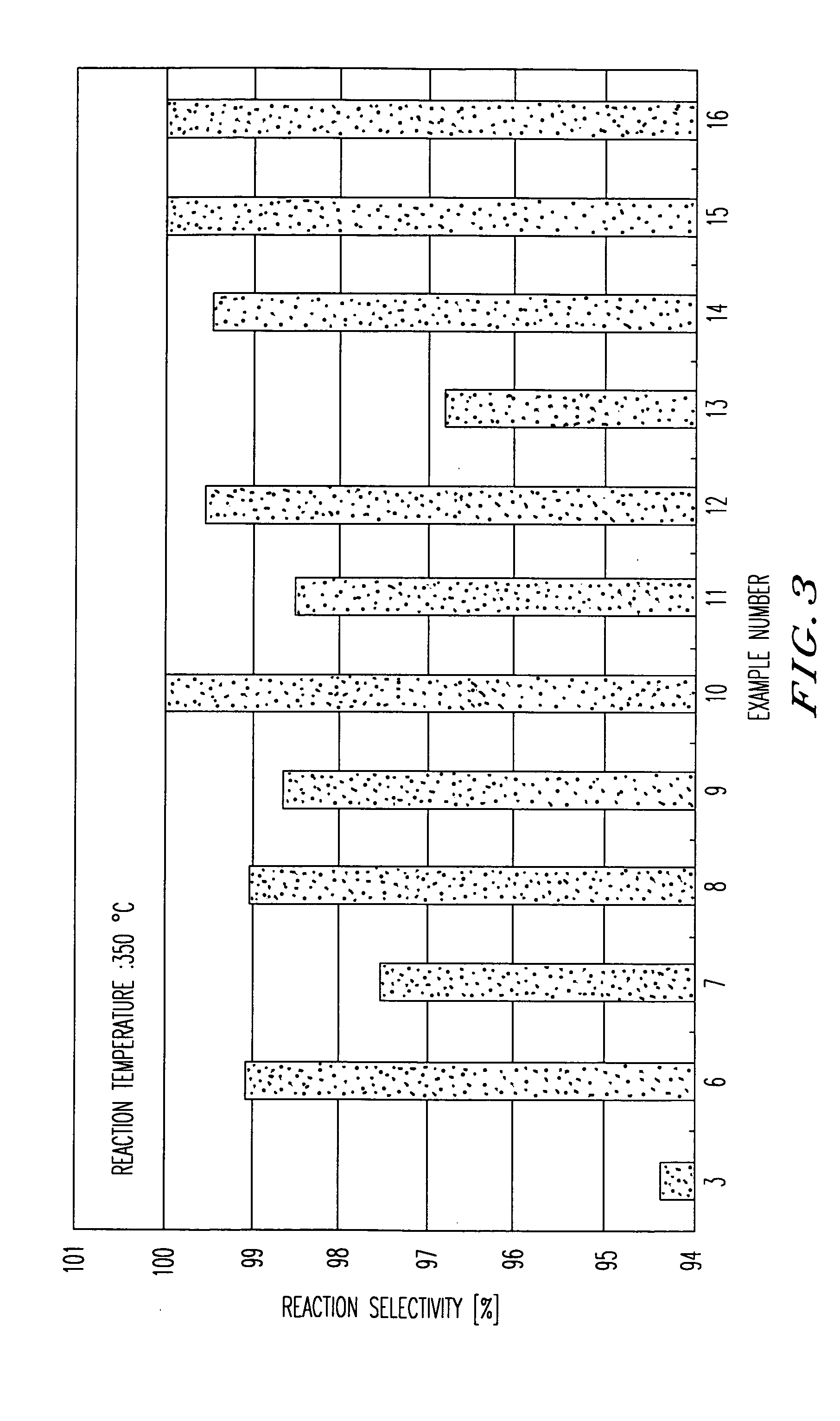
[0055] The present invention will be concretely explained with reference to examples.
examples 1 to 5
[0056] Using a firing furnace, n-hydrate of zirconium hydroxide (ZrO2.H2O, manufactured by Mitsuwa Chemicals Co. Ltd.) was subjected to a firing treatment wherein it was heated to a temperature of 500° C. in one hour in an air flow of 60 ml / min., and kept at that temperature for one hour, and thereby zirconium oxide was obtained as a zirconia carrier.
[0057] A predetermined amount of the obtained zirconia carrier was charged into an evaporating dish located above a water bath. Pure water was added to the carrier and they are mixed intimately. An aqueous solution of chloroplatinic acid hexahydrate (manufactured by NACALAI TESQE INC.) was added to the evaporating dish, and pure water was further added to reach a predetermined concentration. The dish was located above the water bath while stirring so that the evaporation to dryness of the contents in the dish was carried out, during which a metal salt depositing on a wall of the evaporating dish was washed away with pure water into the...
example 6
[0068] A predetermined amount of the zirconia carrier which was prepared in Examples 1 to 5 was charged into an evaporating dish located above a water bath. Pure water was added to the carrier and they are mixed intimately. An aqueous solution of chloroplatinic acid hexahydrate (manufactured by NACALAI TESQE INC.) and an aqueous solution of lanthanum nitrate hexahydrate (manufactured by Wako Pure Chemicals Industries, Ltd.) were added to the evaporating dish, and pure water was further added to reach predetermined concentrations. The dish was located above the water bath while stirring so that the evaporation to dryness of the contents in the dish was carried out, during which metal salts depositing on a wall of the evaporating dish were washed away with pure water into the bottom of the dish. Such depositing salts arose with proceeding of the water evaporation. The evaporation to dryness took one hour.
[0069] The obtained material through the evaporation to dryness was further drie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com