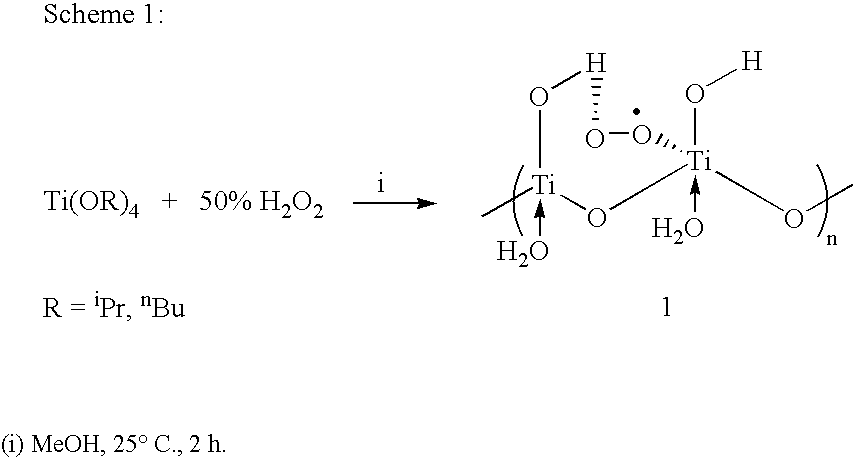
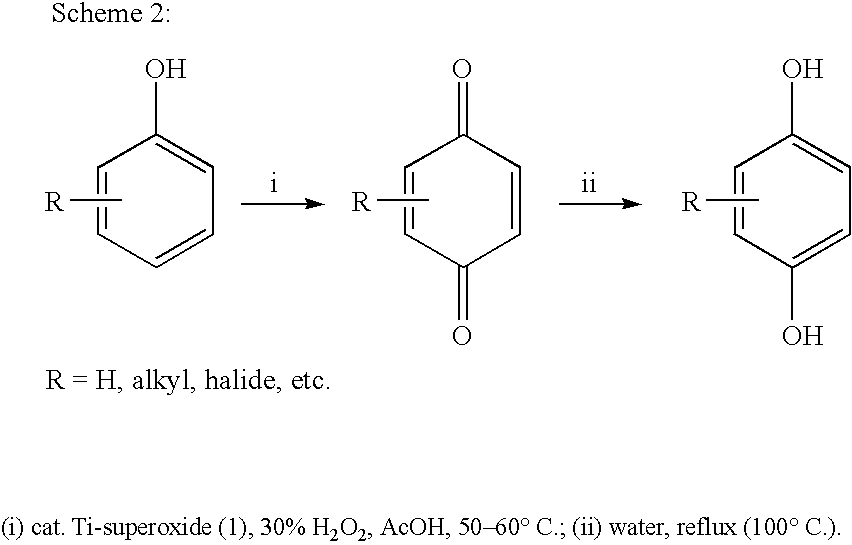
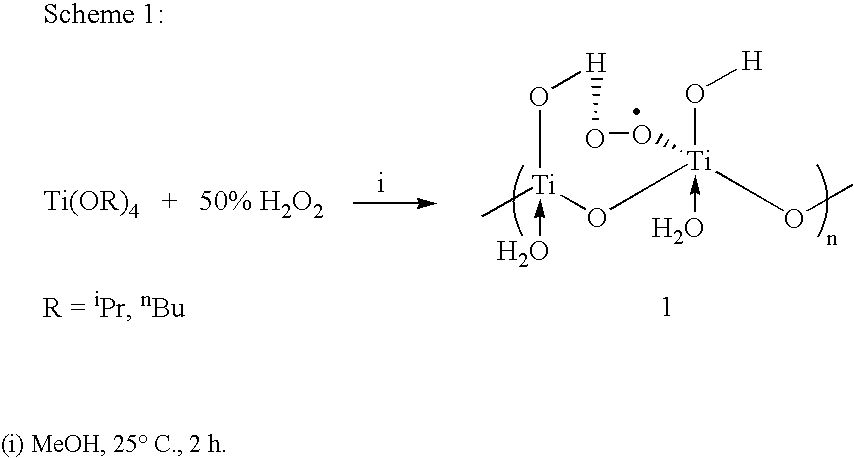
Process for conversion of phenol to hydroquinone and quinones
a technology of hydroquinone and phenol, which is applied in the direction of catalyst activation/preparation, group 3/13 element organic compounds, physical/chemical process catalysts, etc., can solve the problems of increasing the cost of the process, and reducing the efficiency of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 1,4-hydroquinone
A mixture of phenol (5 mmol) and Ti-superoxide catalyst (125 mg, 20% w / w) in acetic acid (5 ml) was heated with stirring at 50-60° C. under inert atmosphere. To this reaction mixture was added aq. 10% H2O2 (20 mmol) drop wise over 15 min. and heated for 2 h. After this, water (5 ml) was added and the reaction mixture was heated to reflux for 8 h. The catalyst was recovered by simple filtration and 1,4-hydroquinone formed (20%) was separated by chromatographic purification.
example 2
Preparation of 1,4-hydroquinone
A mixture of phenol (5 mmol) and Ti-superoxide catalyst (125 mg, 20% w / w) in acetic acid (5 ml) was heated with stirring at 50-60° C. under inert atmosphere. To this reaction mixture was added aq. 50% H2O2 (20 mmol) drop wise over 15 min. and heated for 1 h. After this, water (5 ml) was added and the reaction mixture was heated to reflux for 7 h. The catalyst was recovered by simple filtration and 1,4-hydroquinone formed (61%) was separated by chromatographic purification.
example 3
Preparation of 1,4-hydroquinone
A mixture of phenol (5 mmol) and Ti-superoxide catalyst (125 mg, 20% w / w) in acetic acid (5 ml) was heated with stirring at 50-60° C. under inert atmosphere. To this reaction mixture was added aq. 30% H2O2 (20 mmol) drop wise over 15 min. and heated for 1 h. After this, water (5 ml) was added and the reaction mixture was heated to reflux for 6 h. The catalyst was recovered by simple filtration and 1,4-hydroquinone formed (60%) was separated by chromatographic purification.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com