System and method for continuous data analysis of an ongoing clinical trial
a clinical trial and data analysis technology, applied in the field of clinical trial data processing, can solve the problems of prolonged unnoticed treatment toxicity, skewing the data and outcome of the clinical trial, and no way to separate out subjects and their data into corresponding groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
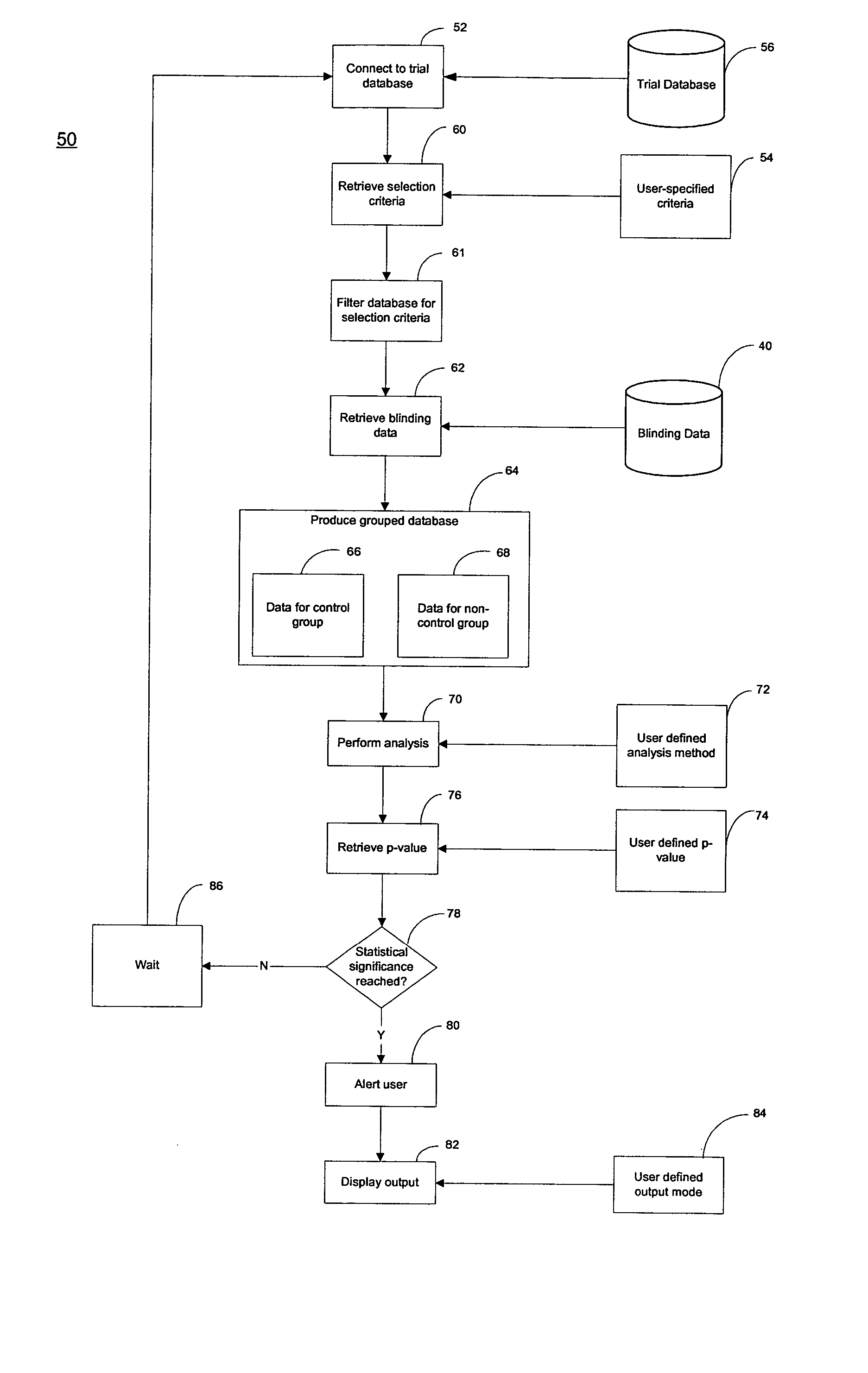
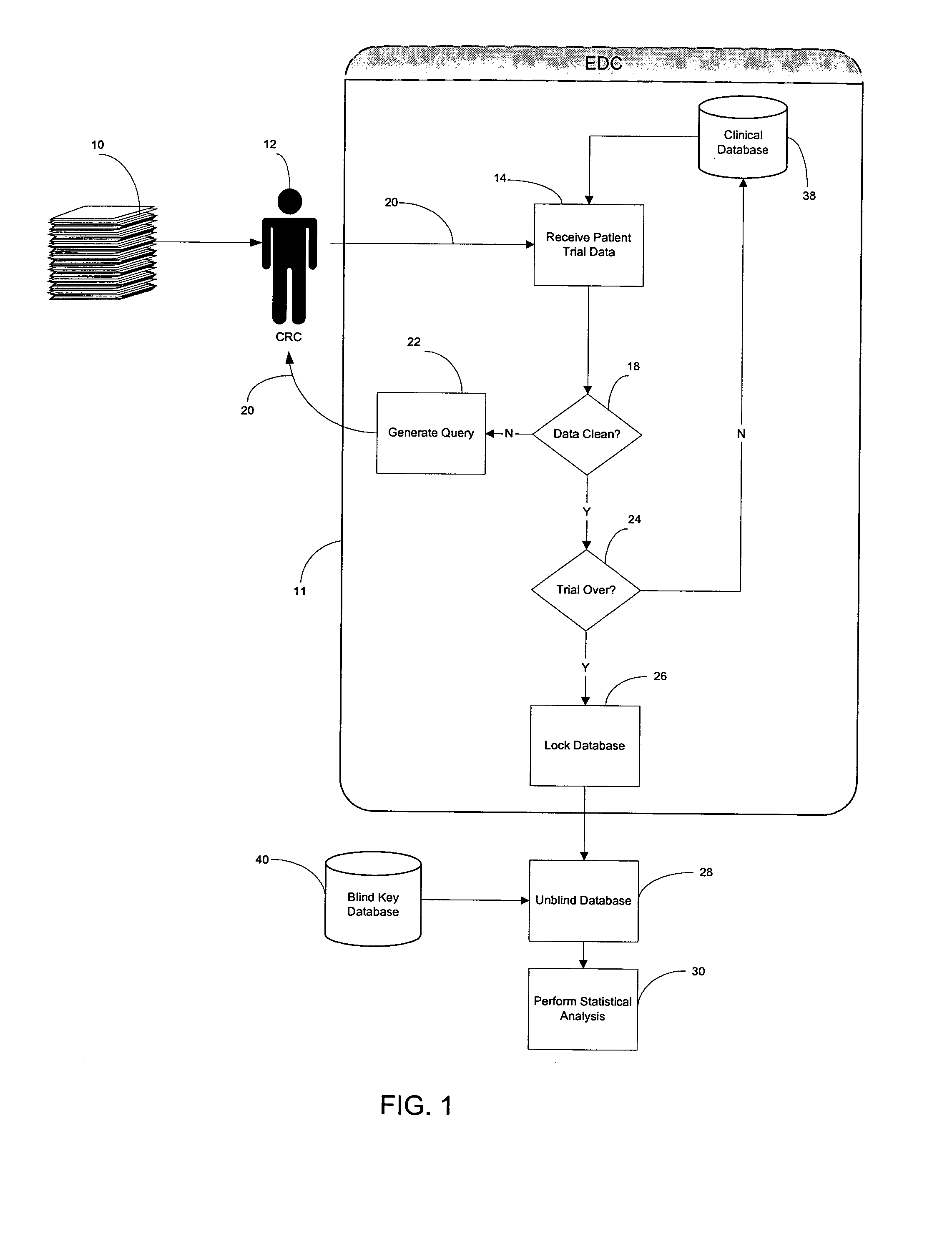
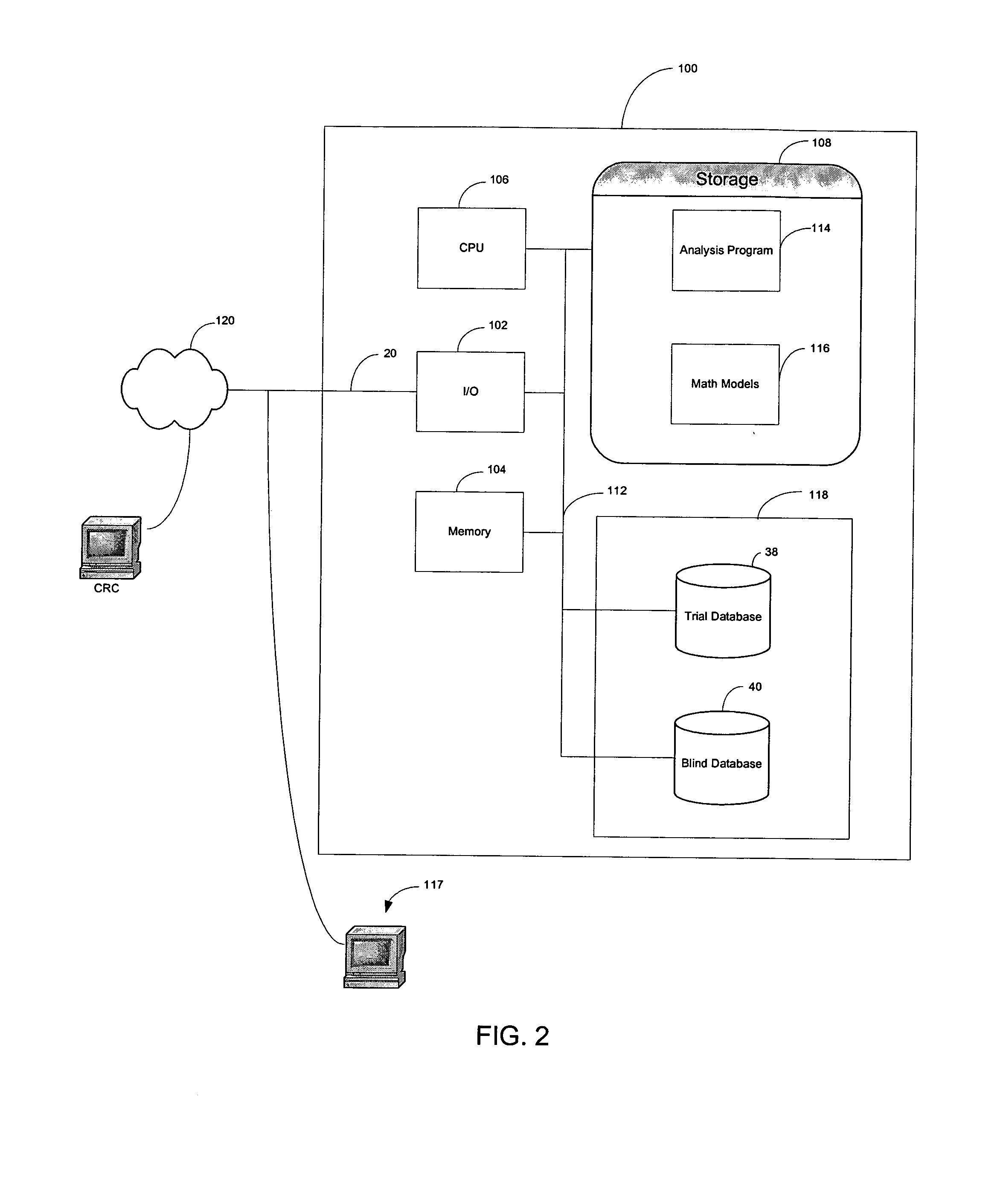
[0060] As shown in FIG. 2, a clinical trial data management system 100 of the present invention is an Internet-enabled application solution framework that automates data collection, data cleaning, grouping if needed (as will be explained more fully later herein) and statistical data analysis while the trial is ongoing. The system 100 is connected to a computer network such as the Internet 120 through, for example, an I / O interface 102, which receives information from and sends information to Internet users over a communication link 20 and to one or more operators using a work station 117. The Internet users are typically CRC's located at various trial sites who transcribe the subjects' charts to the system 100. The system 100 includes, for example, memory 104 which is volatile, processor (CPU) 106, program storage 108, and data storage device 118, all commonly connected to each other through a bus 112. The program storage 108 stores, among others, a clinical trial analysis program o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com