Novel antibody delivery system for biological response modifiers
a biological response modifier and antibody technology, applied in the field of monoclonal antibodies and biological response modifier conjugates, can solve the problems of inability to use specific delivery systems for targeting tissues or cells, inability to conjugate antibodies to biological response modifiers such as tumor necrosis factor, and insufficient prior art to treat a wide variety of human cancers, etc., to achieve the effect of reducing damage or injury, and reducing the risk of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
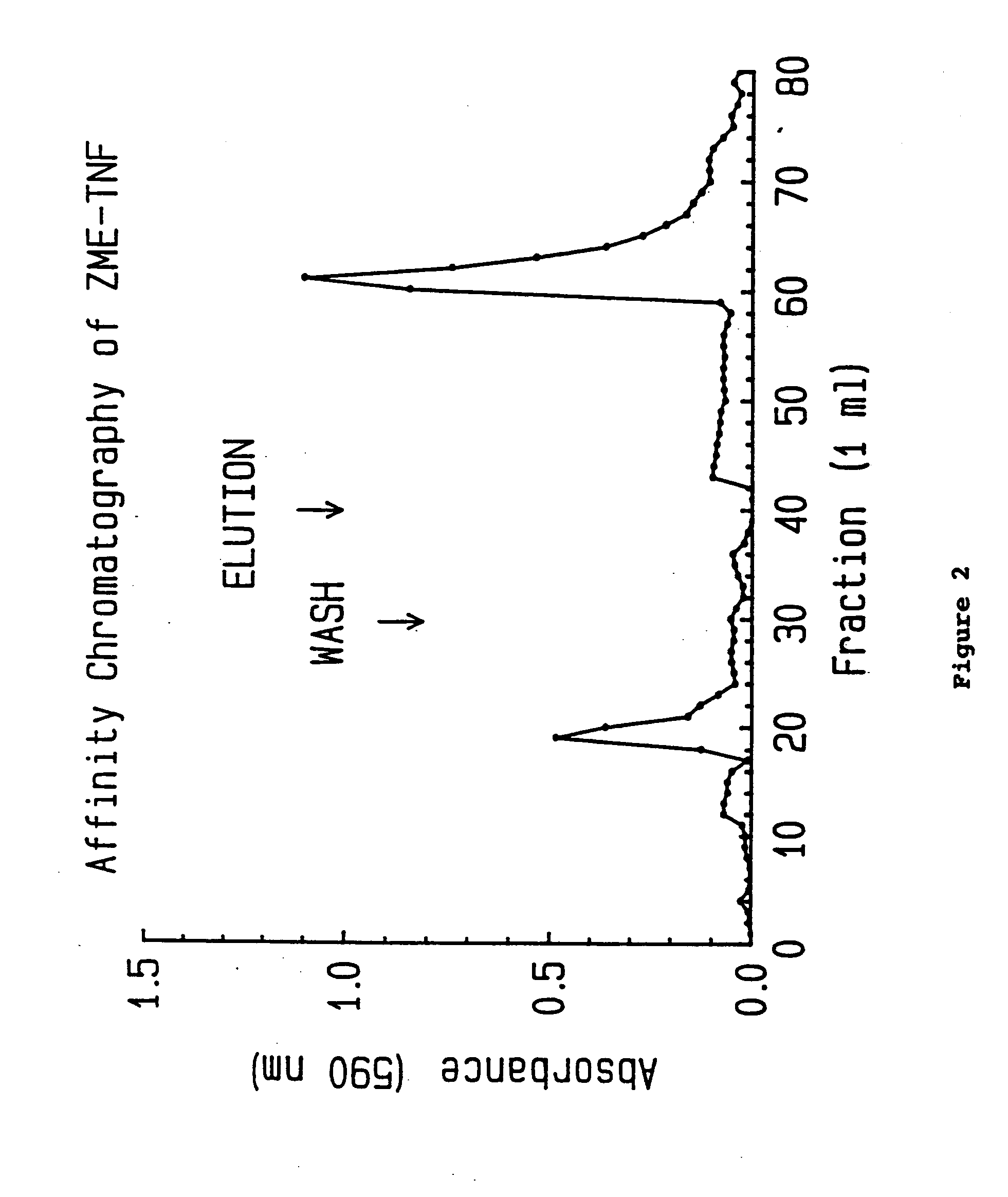
Purification of TNF
[0055] TNF may be purified by techniques known to these in the art. For instance, Aggarwal et al. describes the purification of TNF from various sources including several cell lines of monocytic origin. Aggarwal (1986) Methods of Enzymology 116:448, incorporated herein by reference. This method may also be utilized to purify TNF from other sources.
[0056] TNF is preferably obtained by recombinant technology known to those of skill in the art. Such a preparation is, for example, described in detail in U.S. Pat. No. 4,677,063 and European Publication EP 186,214. The TNF preparation used to obtain the following data depicted in the following Examples was obtained from Genentech Corp., South San Francisco, Calif. The human recombinant DNA derived material was purified to homogeneity from extracts of E. coli. TNF migrated as a single band with an approximate molecular weight of 18,000 daltons.
example 2
Assay of TNF Cytotoxic Activity
[0057] The TNF cytotoxic activity was monitored utilizing the following assay on L-929 cells. Forty thousand murine L-929 fibroblasts in MEM media containing 10% FCS were added to each well of a 96 well plate and incubated 24 hours at 37° C. (5% CO2). Cells were then treated with various amounts of either TNF or TNF-antibody conjugate in medium containing 0.5 g / ml Actinomycin-D for 24 hrs at 37° C. (5% CO2). The cells were washed with phosphate buffered saline (PBS), pH 7.2 and viable cells were stained with crystal violet. The plates were read at 590 nm to determine viable cell number. TNF with a specific activity no lower that 1×107 U / mg was used for conjugation with the antibodies. A unit of TNF activity is the amount of TNF protein which causes 50% inhibition of L-929 cell growth.
example 3
Modification of TNF with Iminothiolane
[0058] TNF in phosphate buffered saline was concentrated to approximately 2 milligrams / ml in a Centricon 10 microconcentrator. Triethanolamine hydrochloride (TEA / HCl), pH 8.0 and EDTA were added to a final concentration of 60 mM TEA / HCl and 1 mM EDTA pH 8.0. 2-Iminothiolane stock solution (20 mM) was added to a final concentration of 1 mM and the sample was incubated for 90 minutes at 4° C. under a stream of nitrogen gas.
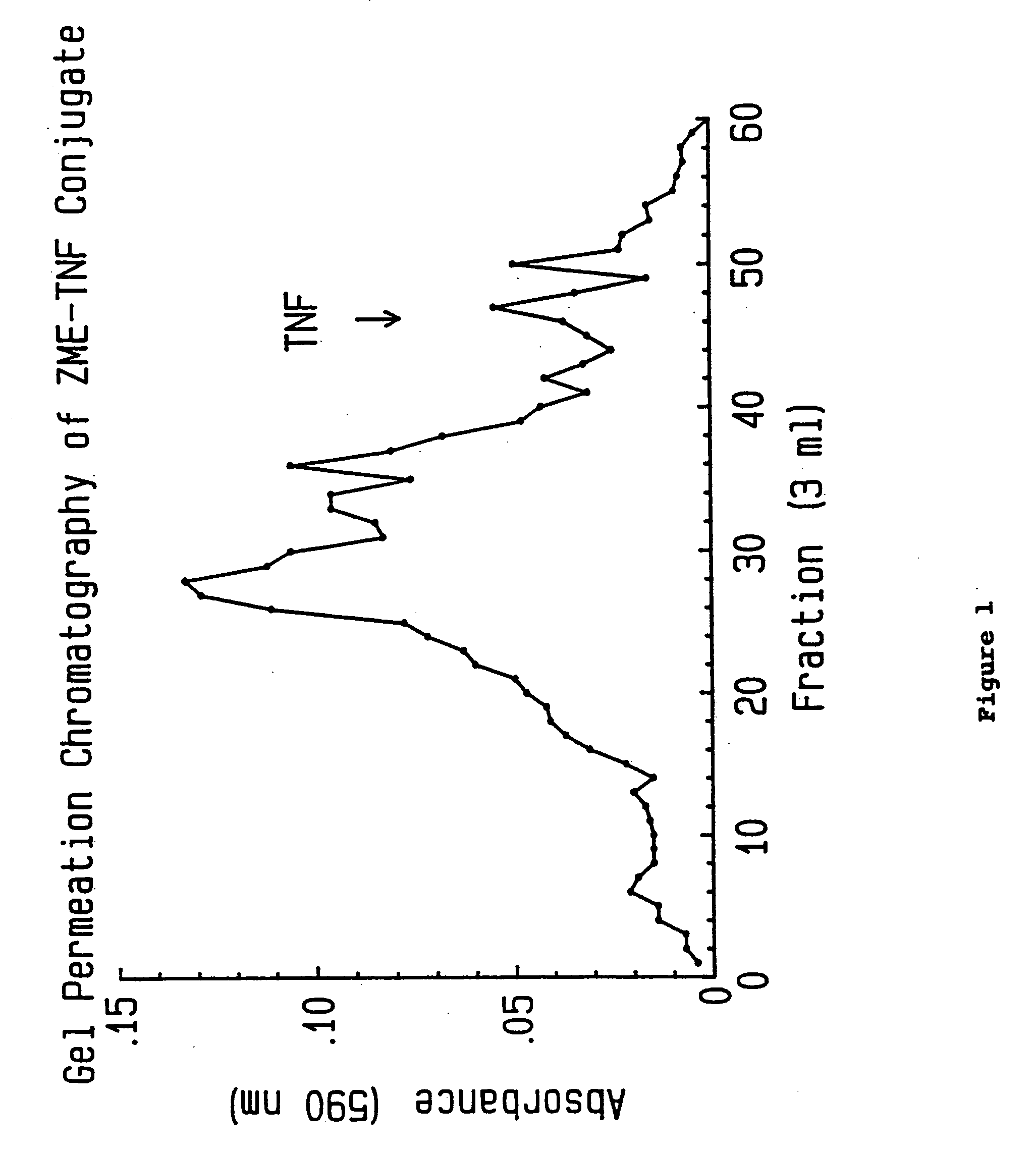
[0059] Excess iminothiolane (IT) was removed by gel filtration on a column of Sephadex G-25 (1×24 cm) pre-equilibrated with 5 mM bis-tris / acetate buffer, pH 5.8 containing 50 mM NaCl and 1 mM EDTA. Fractions were analyzed for protein content in microtiter plates using the Bradford dye binding assay. Briefly, forty microliters of sample, 100 ul of phosphate buffered saline (PBS) and 40 ul of dye concentrate were added to each well. Absorbance at 600 nm was read on a Dynatech Microelisa Autoreader. TNF elutes at the void volume...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Biological properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com