Microfluidic chip or biomolecule crystallization
a microfluidic chip and biomolecule technology, applied in the field of microfluidic systems and methods for crystallizing biomolecules, can solve the problems that the prior crystallization devices available for high throughput screening of crystallization conditions cannot address at least some of the features listed below, and achieve the effect of promoting crystal growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
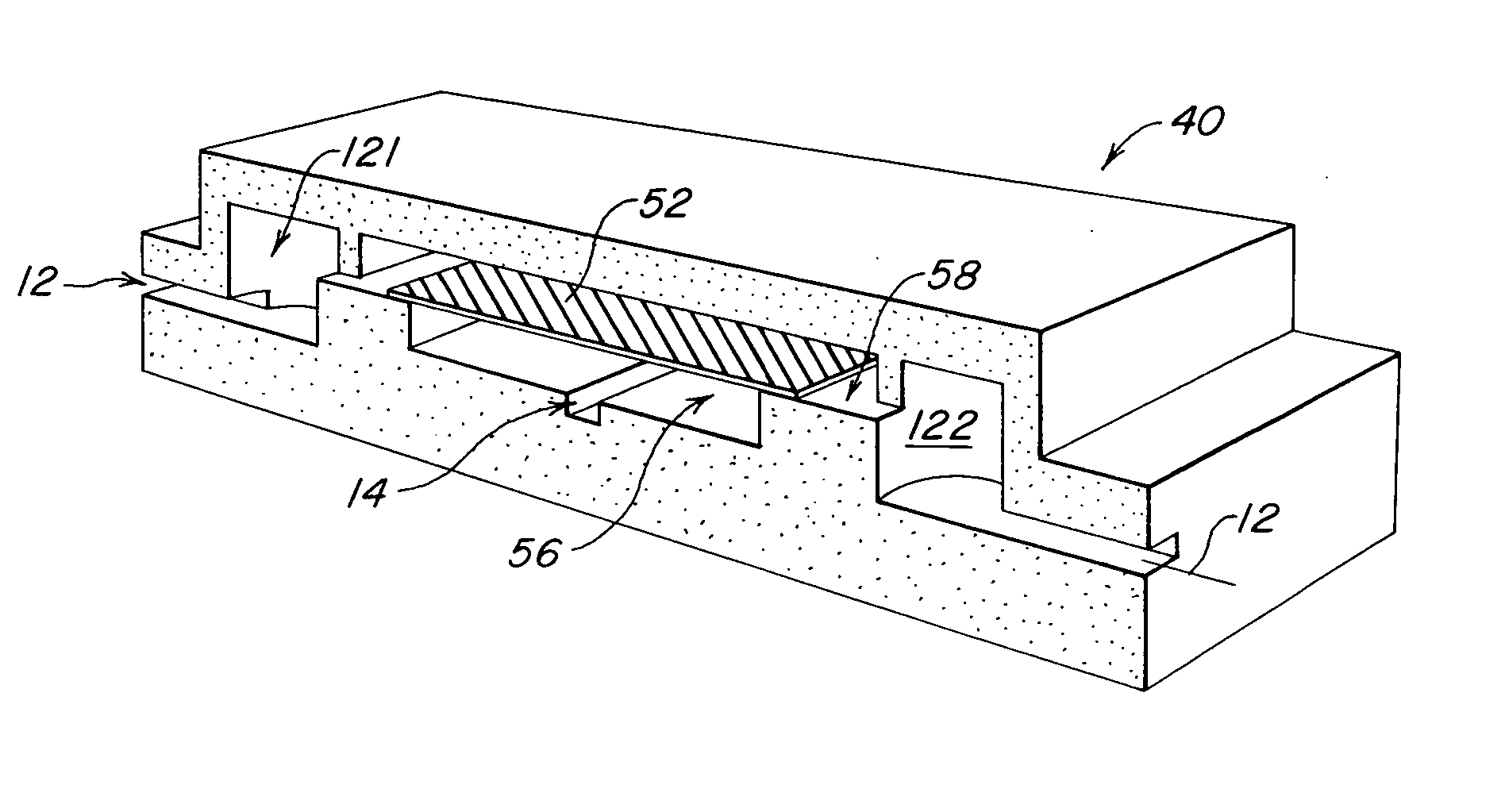
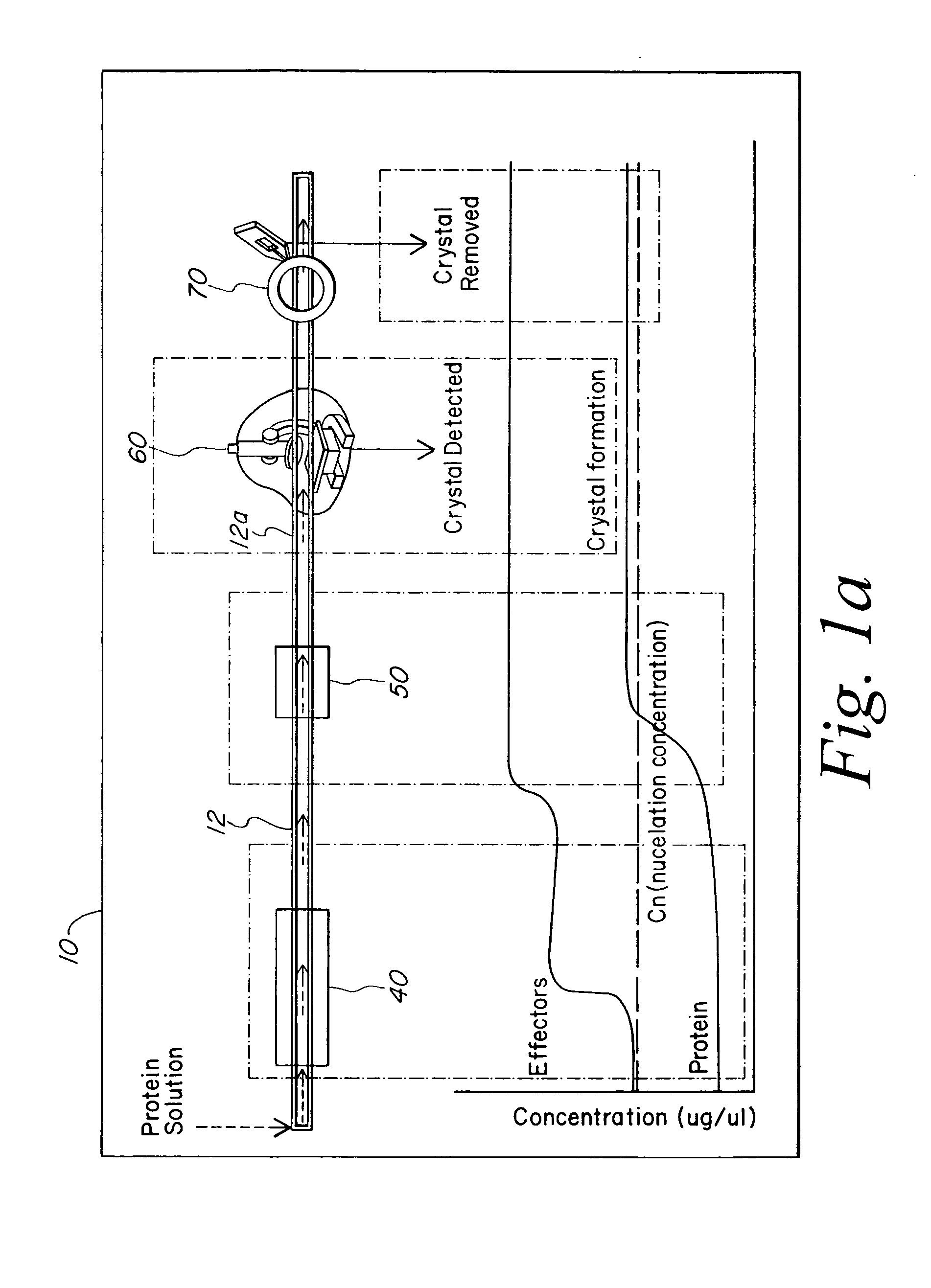
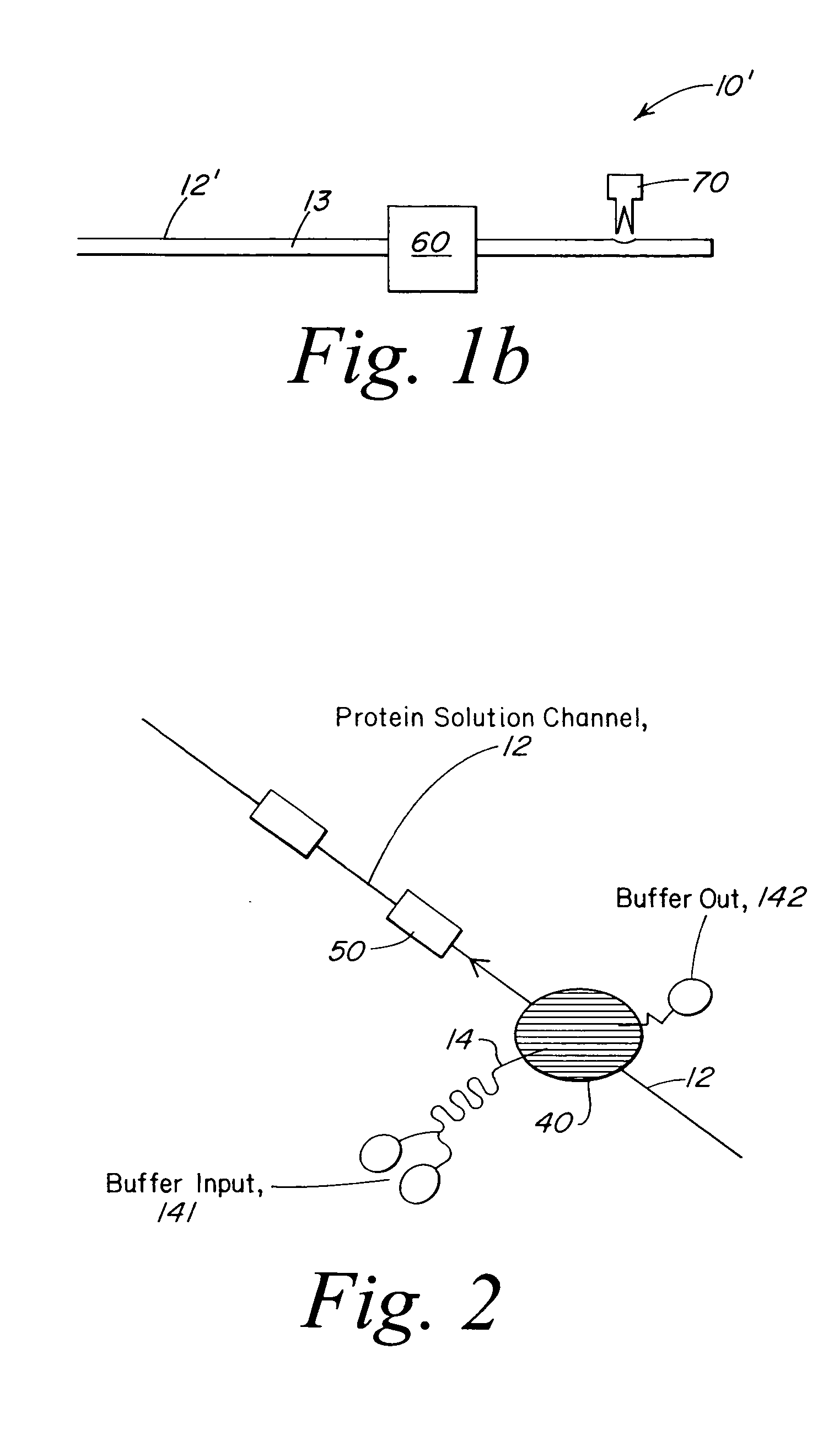
[0021] The present invention provides a microstructured crystallization system that can be formed on a microfluidic chip. The crystallization system may be used to make single crystals from nanogram quantities of protein or other biomolecule, or to test 600-1000 crystallization conditions from microgram quantities of protein or other biomolecule. The present invention will be described below relative to an illustrative embodiment for crystallizing proteins. Those skilled in the art will appreciate that the present invention may be used to crystallize any desired biomolecule or create co-crystals of any combination of biomolecules and may be implemented in a number of different applications and embodiments. The present invention is not specifically limited in its application to the particular embodiments depicted herein.
[0022] The biomolecule crystallization system of the present invention integrates conventional membrane filter technology into a microfluidic chip to perform microdi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com