Compounds and their uses
a technology applied in the field of compounds and their uses, can solve the problems of rapid turnover rate, severe depletion of nad in cells suffering from massive damage, and immediate activation of parp by up to 500-fold, so as to prevent and/or ameliorate the effects of conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
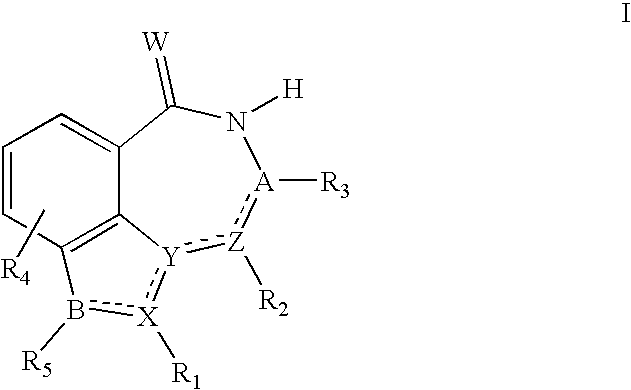
[0131] Compounds of the following general formula I-1 may be prepared, for example, by the following methods.
[0132] Scheme 1 below illustrates schematically the preparation of Example compounds 1 through 6.
[0133] Step 1
[0134] Methyl indole-4-caboxylate 1 (7.2 g, 41.09 mmol commercially available) was dissolved in dry CH2Cl2 (220 mL). To this stirred solution was added ZnCl2 (11.53 g, 84.64 mmol). The mixture was cooled down to 0° C. and added slowly CH3MgBr (5.39 g, 45.19 mmol). It was stirred at 0 C for 15 min. and at room rt. for 1 h. To this mixture was added chloro acetyl chloride and stirred for 2 h. AlCl3 was then added and the mixture was run for an additional 18 h. The reaction mixture was cooled to 0° C. and washed with cold water (60 mL) and extracted with some additional CH2Cl2 (2×60 mL). The organic layer was washed with brine, dried over MgSO4, and concentrated to dryness. The crude solid residues were triturated in ether to give a nice off-white solid 2 (5.1 g) wh...
example 1-1
[0139]
[0140] The compound was prepared as described in Scheme 1. 1H NMR (400 Hz, d6-DMSO) 11.84 (s, 1H), 10.22 (s, 1H), 7.87 (s, 1H), 7.54 (m, 2H), 7.19 (t, 1H, J=7.7 Hz), 3.57 (m, 4H), 3.34 (m, 2H), 2.44 (bs, 4H). Anal. (C15H16N4O2), C H N.
[0141] The HCl salt form:. The cyclic morpholine (0.35 g, 1.23 mmol) was dissolved in THF (10 mL). To the solution was added slowly 4 M HCl in dioxane (1.41 mmol, 0.35 mL). The amine salt was crashed out of the solution which was collected by vacuum filtration. The solid material was quickly transferred to a storage vial due to its hygroscopic in nature. The product is a nice yellow solid (0.16 g). Mp.=238-240° C. 1H NMR (400 Hz, d6-DMSO) 12.33 (s, 1H), 10.53 (s, 1H), 10.30 (s, 1H), 7.98 (d, 1H, J=2.9 Hz), 7.60 (m, 2H), 7.25 (t, 1H, J=7.8 Hz), 4.29 (s, 2H), 3.95 (s, 2H), 3.80 (bs, 2H), 3.56 (bs, 2H), 3.25 (bs, 2H). Anal. (C15H16N4O2.1HCl), C H N.
example 2-1
[0142]
[0143] The compound was prepared as described in Scheme 1. Mp.=232-236° C. 1H NMR (400 Hz, d6-DMSO) 11.88(s, 1H); 10.27(s, 1H); 7.91(s, 1H); 7.60-7.58(m, 2H); 7.23(t, 1H, J=7.84 Hz); 3.36(s, 2H); 2.56(s, 4H); 2.38(s, 4H); 2.20(s, 3H); Anal. (C16H19N5O), C H N.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com