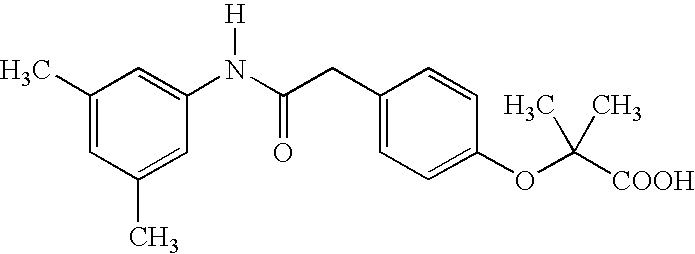
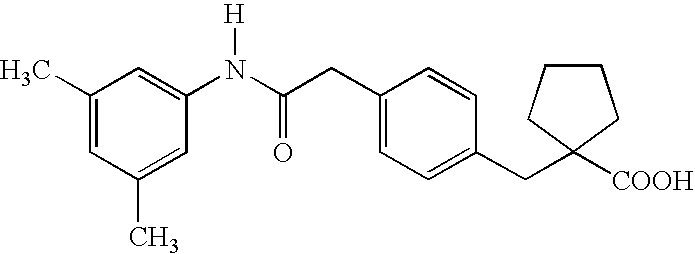
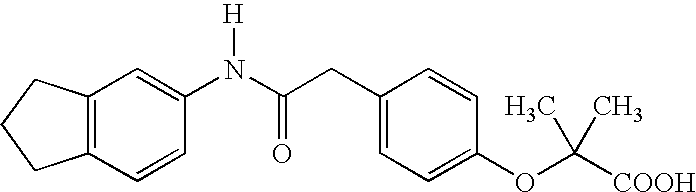
Substituted chiral allosteric hemoglobin modifiers
a technology of allosteric effectors and modifiers, which is applied in the field of substitution of chiral allosteric effectors, can solve the problems of limited clinical potential of these agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Scheme 2 Illustrates a Reaction Scheme for Preparing 4-[[(3,5-dimethylanilino)carbonyl]methyl]phenol, a Compound that is Useful as a Precursor in the Preparation of Some of the Table II Compounds
[0024] A mixture of 4-hydroxyphenylacetic acid (20.0 g, 131 mmol) and 3,5-dimethylaniline (15.9 g, 131 mmol) in xylene (100 mL) was stirred for three days at 160° C. with a Dean Stark trap. The mixture was cooled to room temperature and filtered. The solid product obtained was washed with hexane (200 mL), 10% sodium bicarbonate solution (250 mL), water (200 mL), 10% hydrochloric acid (200 mL), and then water (200 mL). The beige solid was air dried to yield 27.7 g, 82.7%. mp 183-185° C.
[0025]1H NMR (CDCl3): δ2.25 (s,6H), 3.60(s,2H), 6.71(s,1H), 6.82(d,2H,J=8.5 Hz), 7.05 (s,2H), 7.13 (d, 2H, J=8.4 Hz).
[0026] Anal: C16H17NO2; Calculated C, 75.27; H, 6.71; N, 5.49; Found C, 75.18, H, 6.69 and N, 5.36
1-[4-[[(3,5-dimethylanilino)carbonyl]phenoxy]-3-methylcyclopentane carboxylic Acid (3)
[0027]...
example 2
[0240] Radiation Oncology. Solid tumors, such as brain metastasis and lung cancers, are oxygen deficient masses. The allostcric effectors of this invention deliver more oxygen to tumors, which increases radical formation that increases tumor killing during radiation.
example 3
[0241] Hypothermia limiting or preventing hypoxia induced irreversive myocardial damage. The allosteric effectors increase the efficiency of oxygen delivery at low blood flow and low temperatures, thus having the ability to prevent myocardial damage.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com