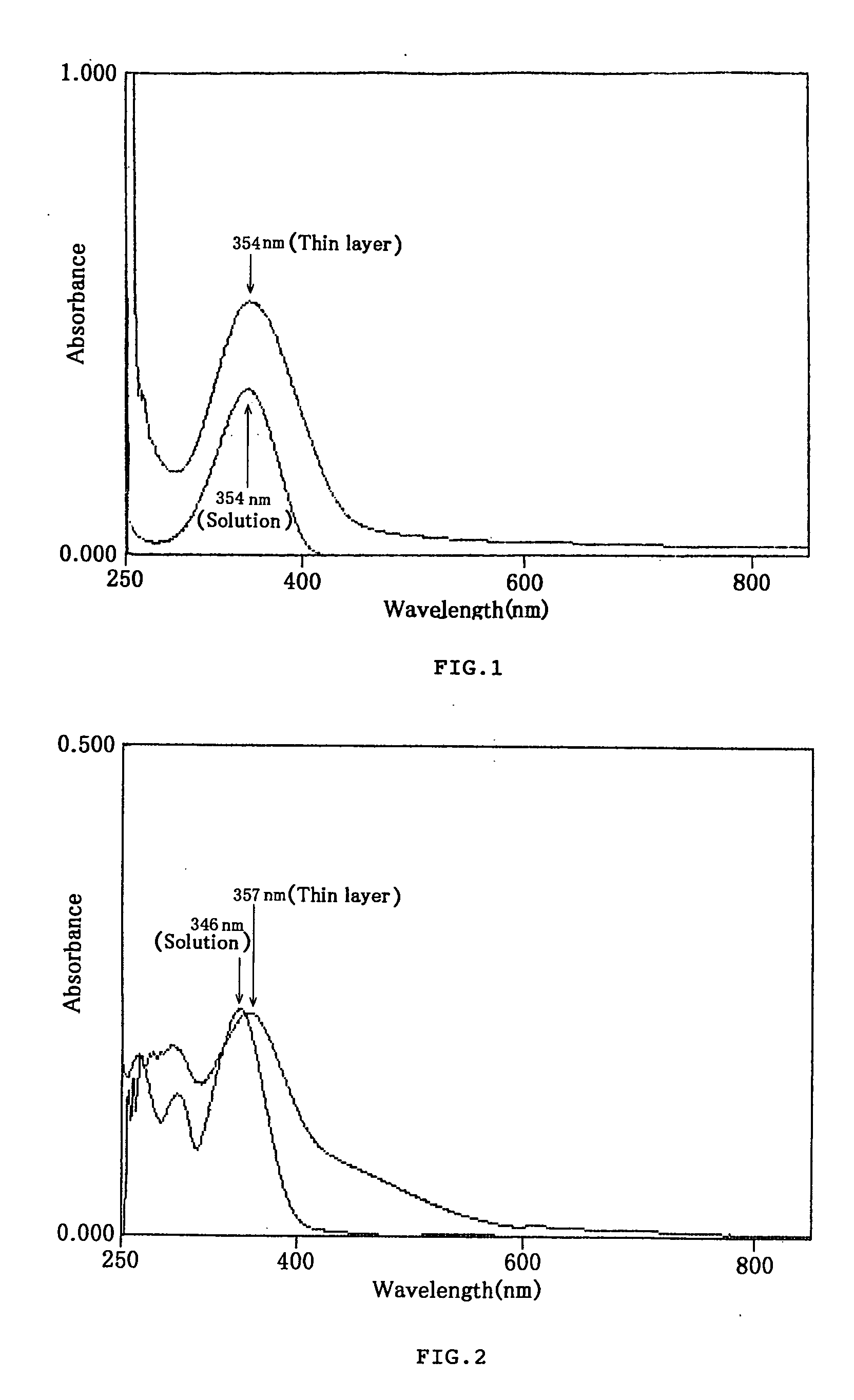
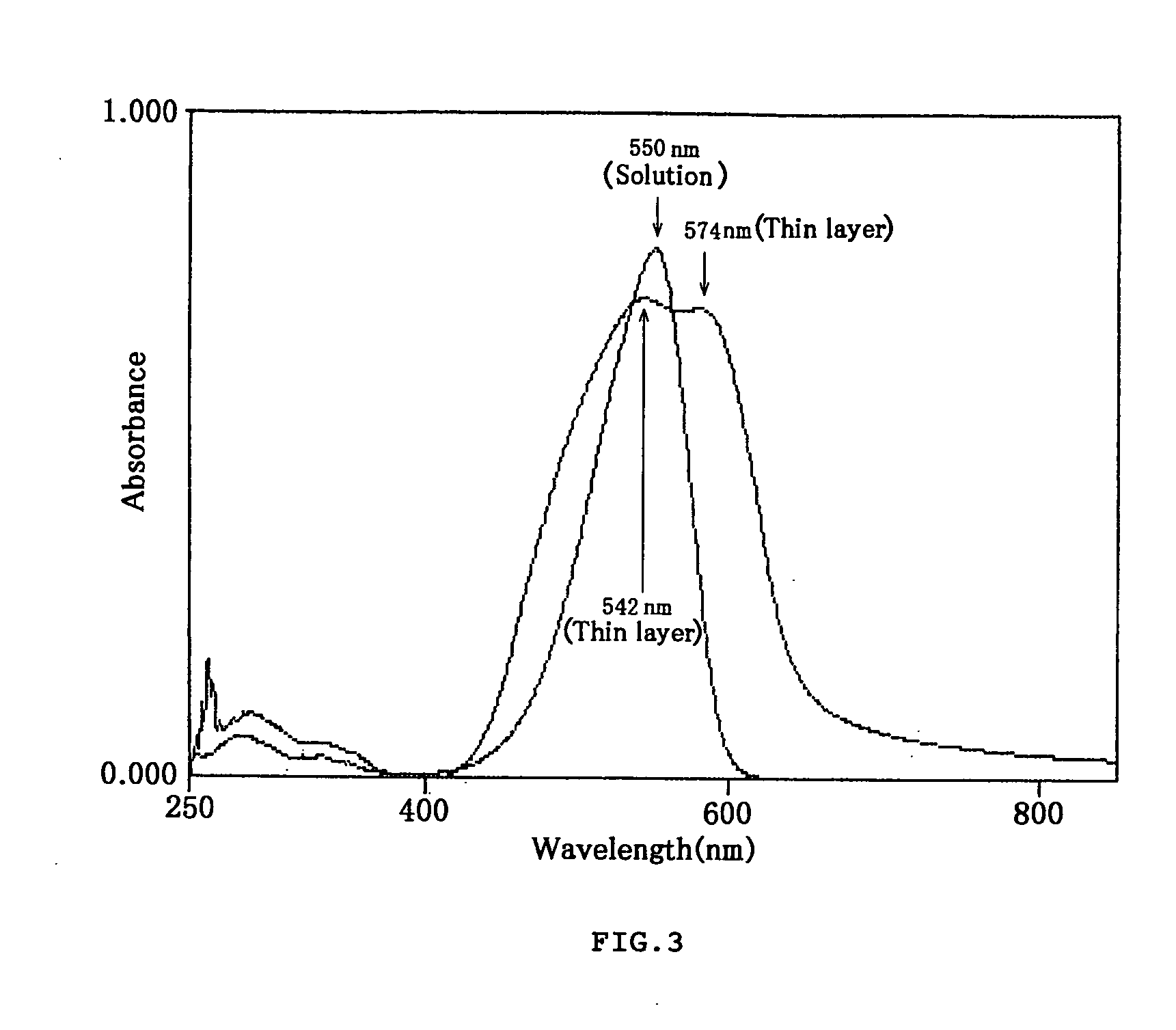
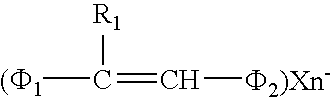Styryl dyes
a technology of styryl dyes and dyes, which is applied in the field of organic dye compounds, can solve the problems of low storage density of organic dyes, inability to meet the high-density requirements of many fields, and easy change of recording layers in environmental ligh
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Styryl Dye
[0040] Twenty milliliters (ml) of acetic anhydride and 0.6 ml of triethylamine were placed in a reaction vessel, and mixed with 4 g of 2,3,4-trimethylthiazolium=iodide and 2 g of 4-cyanobenzaldehyde. The resulting mixture was reacted at 80° C. for one hour under stirring conditions, and then was cooled down. The crystals formed were collected, washed with ethanol, dissolved in a solution of methanol and chloroform under heating, and then filtered. The obtained filtrate was distilled to remove chloroform and cooled down to obtain 1.1 g of a yellow crystal of the styryl dye represented by Chemical Formula 9. When measured in a conventional manner, the melting point of the crystal was 261-263° C.
[0041] The styryl dye of this Example with remarkable optical properties has various uses in many fields including those of optical recording media as a light absorbent.
example 2
Styryl Dye
[0042] Forty milliliters of ethanol and 2 ml of triethylamine were placed in a reaction vessel, and mixed with 6 g of 1,2,3,3-tetramethyl-3H-indolenium=tosylate and 3 g of 3-nitrobenzaldehyde. The resulting mixture was reacted at 80° C. for one hour under stirring conditions, and then was cooled down. The crystals formed were collected, dissolved in a solution of methanol and chloroform under heating conditions, and then filtered. The obtained filtrate was distilled to remove chloroform and cooled to obtain 3.4 g of an orangish yellow crystal of the styryl dye represented by Chemical Formula 40. When measured in a conventional manner, the melting point of the crystal was 233° C.
[0043] The styryl dye of this Example with remarkable optical properties has various uses in many fields including those of optical recording media as a light absorbent.
example 3
Styryl Dye
[0044] Twenty milliliters of ethanol and a very small amount of piperidine were placed in a reaction vessel, and mixed with 5 g of 1,2-dimethylquinolium=iodide and 2.2 g of 2-formylpyridine. The resulting mixture was reacted at 80° C. for 20 min under stirring conditions, and then was cooled down. The crystals formed were collected and recrystallized in ethanol to obtain 1.3 g of an orangish yellow crystal of the styryl dye represented by Chemical Formula 34. When measured in a conventional manner, the melting point of the crystal was 199-200° C.
[0045] The styryl dye of this Example with remarkable optical properties has various uses in many fields including those of optical recording media as a light absorbent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com