Refining agent and refining method
a technology of refining agent and molten iron, which is applied in the direction of furnace details, furnaces, crucible furnaces, etc., can solve the problems of not being able to exert economic effects, raw materials, metal mg, and high cost, and achieves increased reactivity, high efficiency, and the effect of increasing the rate of mgo changing into mg vapor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
(1) First Embodiment
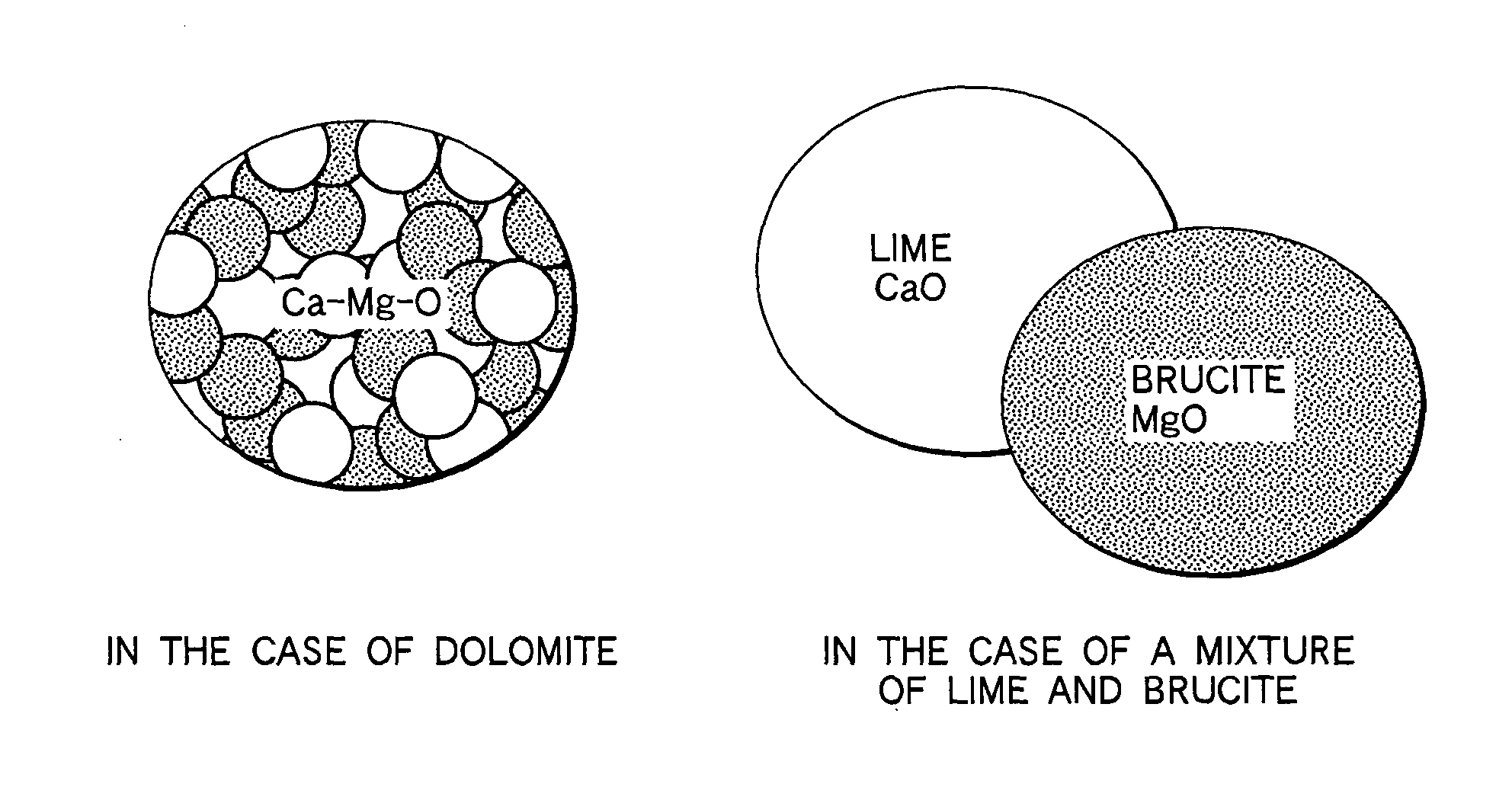
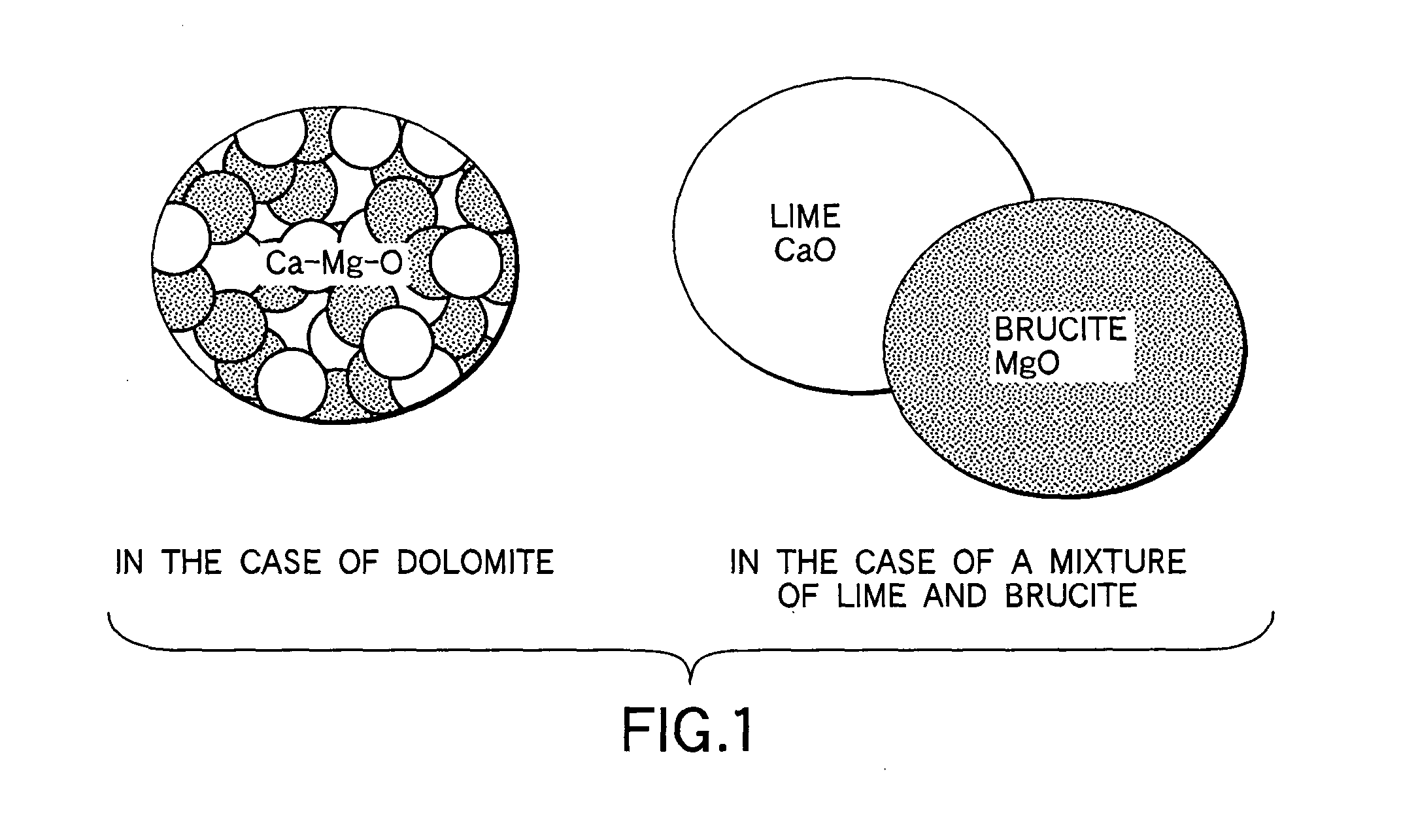
[0046] A refining agent according to the first embodiment of the present invention contains Al, MgO, and CaO as main components, and includes a material, in which MgO and CaO are close to or in contact with each other in a minute state, as a MgO source and CaO source. Typically, it includes dolomite as a MgO source and CaO source. When the refining agent is supplied into molten iron, it produces Mg vapor due to a reaction in the molten iron, and causes a refining reaction by the Mg vapor.
[0047] In the present invention, since CaO is added, Mg vapor is produced in accordance with the following formulas (4) to (7), in place of the formula (3).
6MgO+4Al+CaO→6Mg(g)+CaO.2Al2O3 (4)
3MgO+2Al+CaO→3Mg(g)+CaO.Al2O3 (5)
21MgO+14Al+12CaO→21Mg(g)+12CaO.7Al2O3 (6)
3MgO+2Al+3CaO→3Mg(g)+3CaO.Al2O3 (7)
[0048] In the formula (3), MgO in the starting substance is partly consumed in producing MgO.Al2O3, and thus the ratio of MgO changing into Mg vapor is suppressed to 75% at mo...
second embodiment
(2) Second Embodiment
[0063] A refining agent according to the second embodiment of the present invention contains Al, C, MgO, and CaO as main components. When the refining agent is supplied into molten iron, it produces Mg vapor and a complex oxide of CaO and Al2O3 due to a reaction in the molten iron, and causes a refining reaction by the Mg vapor.
[0064] In the embodiment described above, although Al is used as a reducing agent, Al is relatively expensive. Accordingly, in this embodiment, Al and C are used together as a reducing agent, so that the Al quality is reduced to make flux inexpensive.
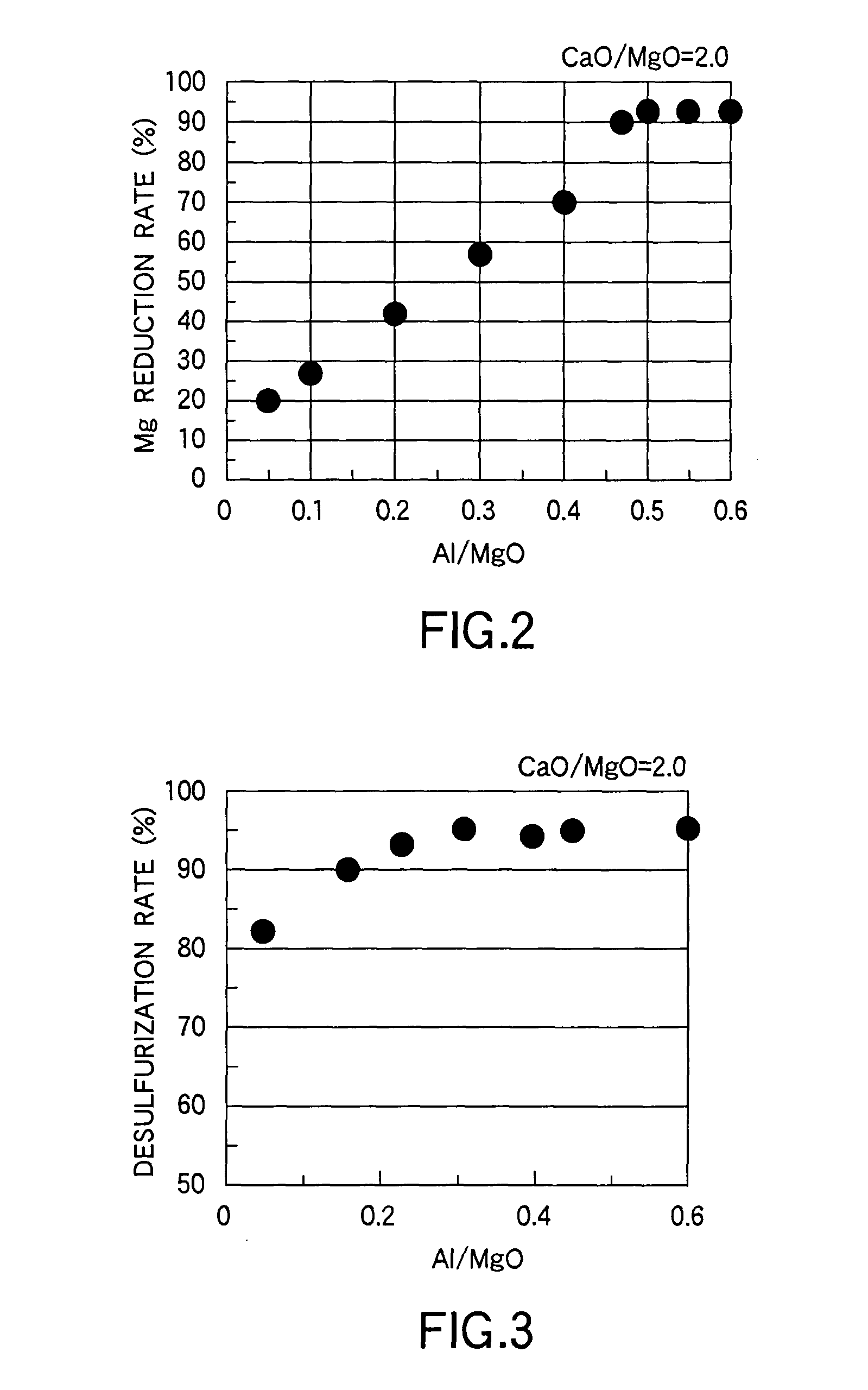
[0065]FIG. 5 is a view showing the relationship between the Al / MgO value, which is on the horizontal axis, of a refining agent, and the Mg reduction rate, which is on the vertical axis, in the case of containing no C, and in the case of adding C(C / MgO=0.3 and 1.0). This result was obtained by adding a refining agent to molten iron at a temperature of 1,300 or more, with CaO / MgO=2.0.
[0066] ...
example 1
[0192] In this example, refining agents according to the present invention and refining agents according to comparative examples were used to desulfurize 200 tons of molten pig iron in a ladle, by a mechanically stirring setup. The molten pig iron used in the treatment was, in advance, subjected to desiliconization treatment at two stages of a runner in a cast house of a blast furnace, and a molten pig iron ladle used as a pig iron-receiving container, following tapping from a blast furnace. With the pre-desiliconization, the molten pig iron composition was set such that [Si]=0.05 to 0.10 mass %, [C]=4.3 to 4.6 mass %, [Mn]=0.22 to 0.41 mass %, [P]=0.10 to 0.13 mass %, and [S] before the treatment=0.040 to 0.042 mass %. The molten pig iron temperature was 1,330 to 1,430° C. Each refining agent according to the present invention was used in a form prepared by mixing and crushing the following materials to have an average particle size 0.6 mm, or a form prepared by pelletizing them. S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com