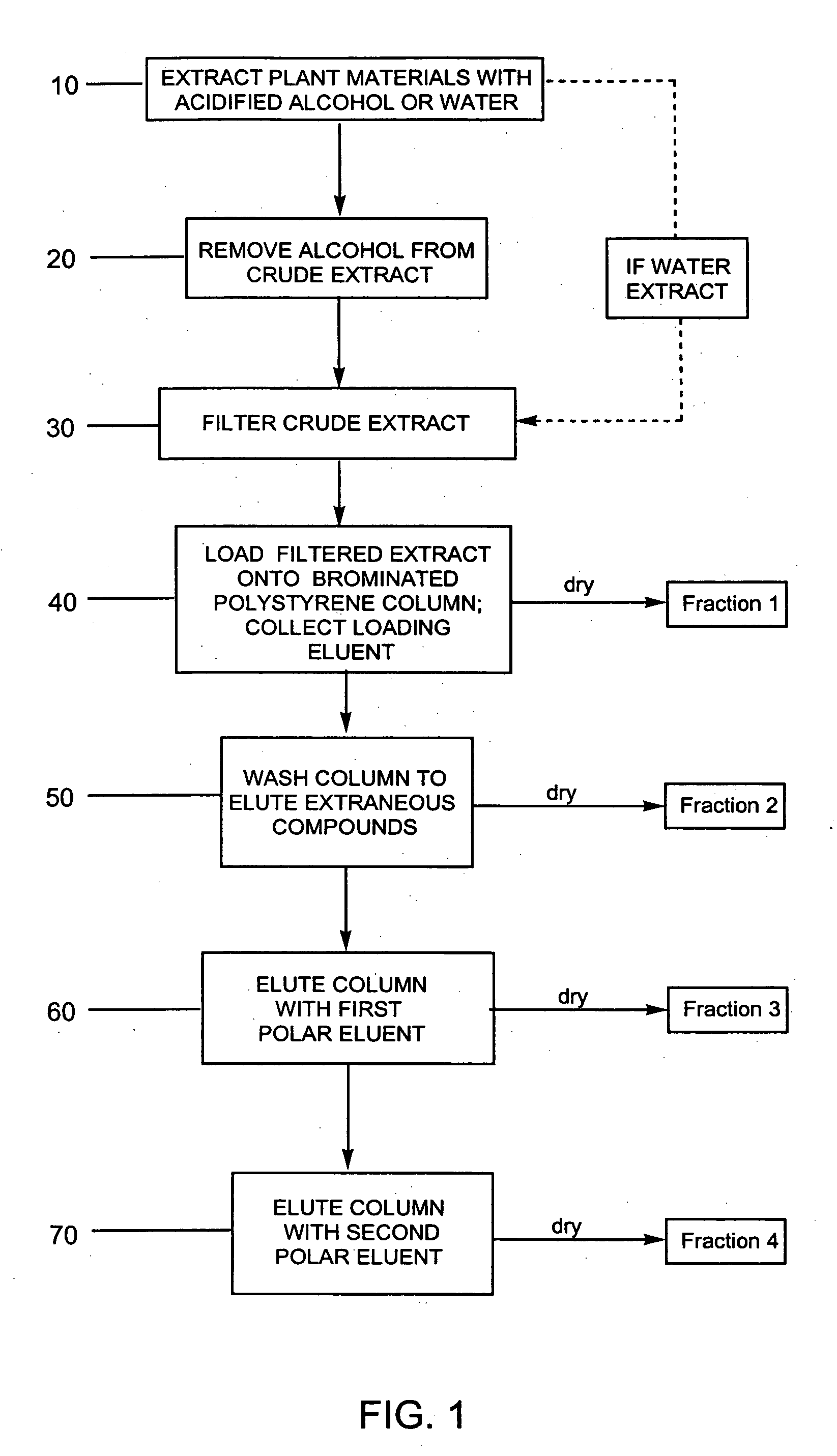
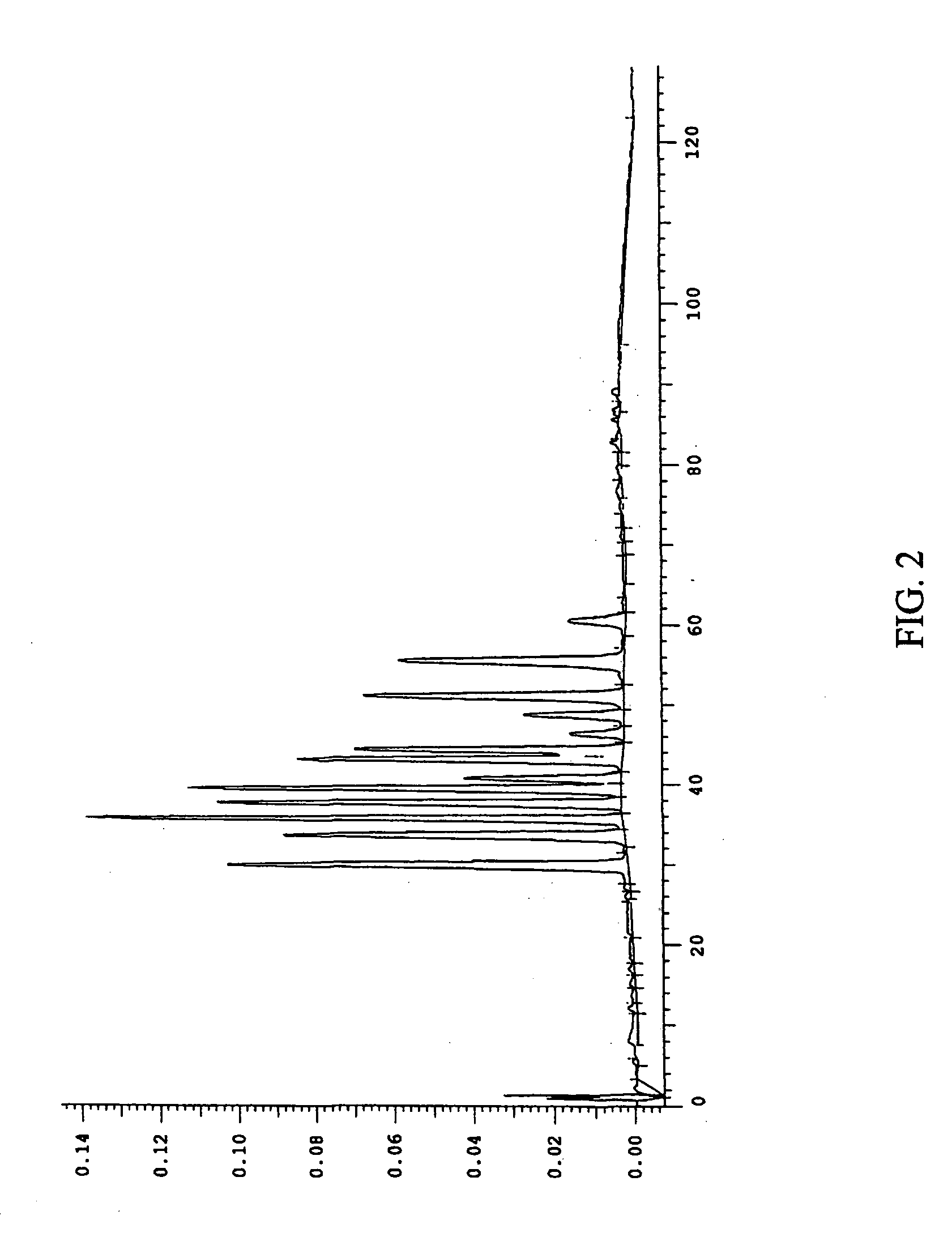
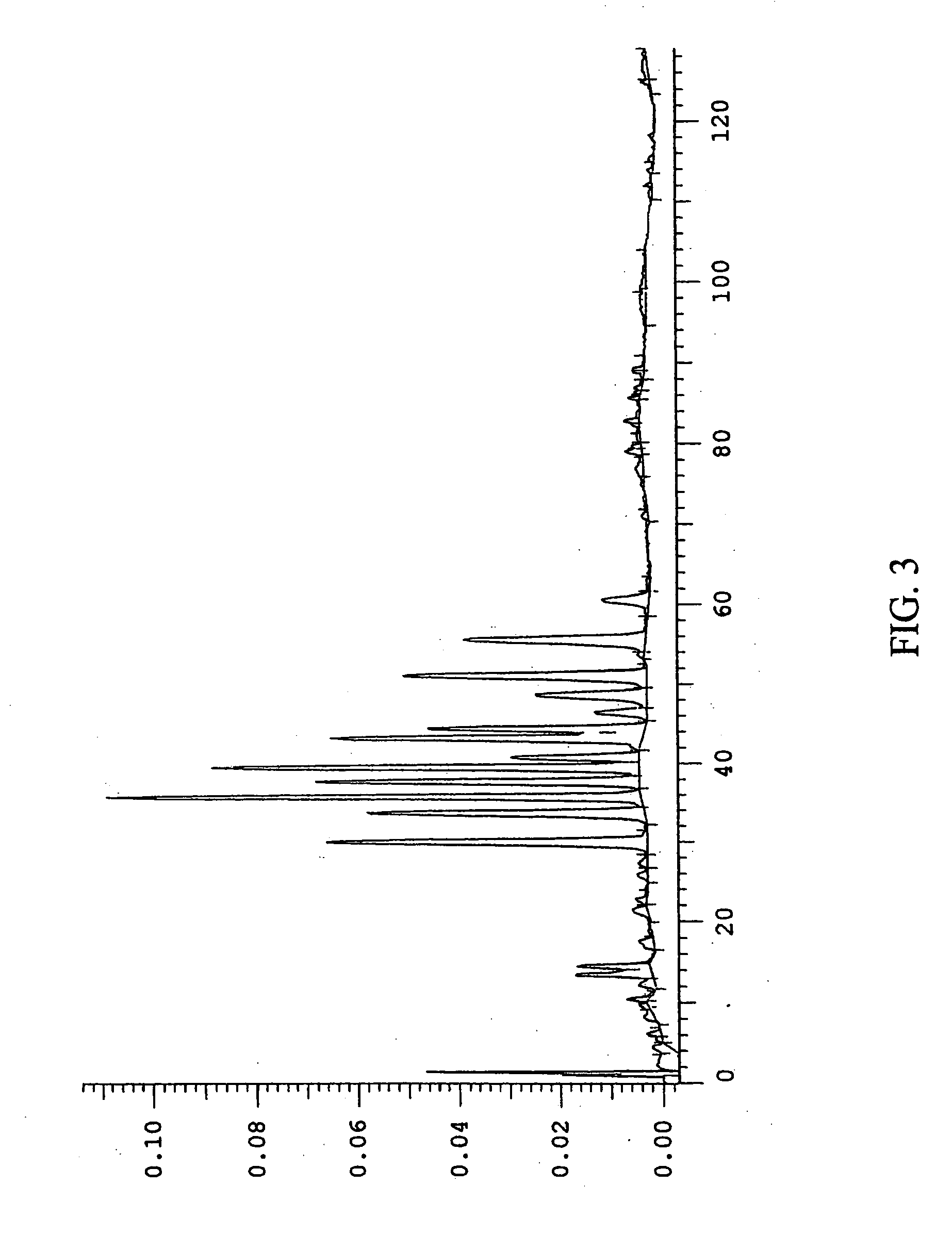
Efficient method for producing compositions enriched in total phenols
a technology of total phenols and compositions, applied in the direction of drug compositions, biocide, plant/algae/fungi/lichens ingredients, etc., can solve the problems of requiring multiple column adsorption, degradation of proanthocyanidins, and the current method of isolating and purifying proanthocyanidins is not easily scaled up to an efficient commercial process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Purification of Bilberry Using a Water Extraction
[0103] Three extractions were performed on 1 kg of dried bilberry raw material. The first extraction used 6 L of water and the other two extractions used 4 L of water. All extractions were acidified with concentrated sulfuric acid to an acid concentration of 5 g / L. There was approximately an 88% recovery of anthocyanins into the crude extract. Exactly 2.3 L of the crude extract were filtered through a 30 micron polypropylene filter with a layer of glass wool over the filter. The glass wool was changed once and the filter rinsed with deionized water. The final volume of the filtrate was 2.43 L with a 90.9% recovery of anthocyanins in the filtrate.
[0104] A column was packed with brominated polystyrene resin SP-207 (Supelco; Belefonte, Pa.) and equilibrated with 0.1% acetic acid. The column was loaded with 2.24 L of the filtrate at a solids concentration of 29.8 g / L using a flow rate of 2.2 mL / min. The loading bleed was less than 0.9% ...
example 2
Purification of Bilberry Using a 70% Ethanol Extraction
[0105] Dried bilberry raw material (667 g), assayed at 2.0% anthocyanins, was extracted by percolation using 70% ethanol / water containing 3% sulfuric acid by volume. The solids in the crude extract contained 3.9% by weight total anthocyanins. One liter of the first extraction volume was mixed with 100 mL deionized water and evaporated in vacuo to about 460 mL to remove the alcohol. Deionized water (300 mL) was added to the mixture, and an additional 170 mL of liquid were evaporated. Deionized water (210 mL) was added to make the final volume 800 mL. To the aqueous mixture was added 150 g of Celite 512 (0.5 to 0.9 g of Celite per gram of solids). The mixture was shaken until homogeneous. The Celite / extract mixture was poured over a 30 g bed of damp Celite 512 under vacuum. Upon completion of filtration, the bed was washed with 1.20 L of 1% aqueous sulfuric acid in 200 mL increments. The filtrate volume was 1855 mL. To the filtra...
example 3
Total Phenol-Enriched Compositions from Blueberries
[0109] To 940 g of dried and ground blueberry (Van Drunen FutureCeuticals; Momence, Ill.) were added 4.0 liters of extraction solvent (1.0% w / v sulfuric acid in 70% ethanol) in a 10 L round bottom flask. The flask was rotated in a constant temperature water bath held at 40° C. for two hours. The mixture was swirled and filtered through a 150 g bed of Celite 512 under vacuum. The blueberry biomass cake was washed with 500 mL of extraction solvent. The cake was carefully scraped away from the Celite bed, poured into a round bottom flask, and re-extracted following the above-described procedure. A third extraction was then performed. The three crude extracts were combined.
[0110] A portion of the combined extracts (2.00 L) was concentrated in vacuo to 175 mL at a water bath temperature of 40° C. The evaporated extract was diluted with deionized water to give 675 mL of crude blueberry extract. The crude extract was loaded without filtr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com