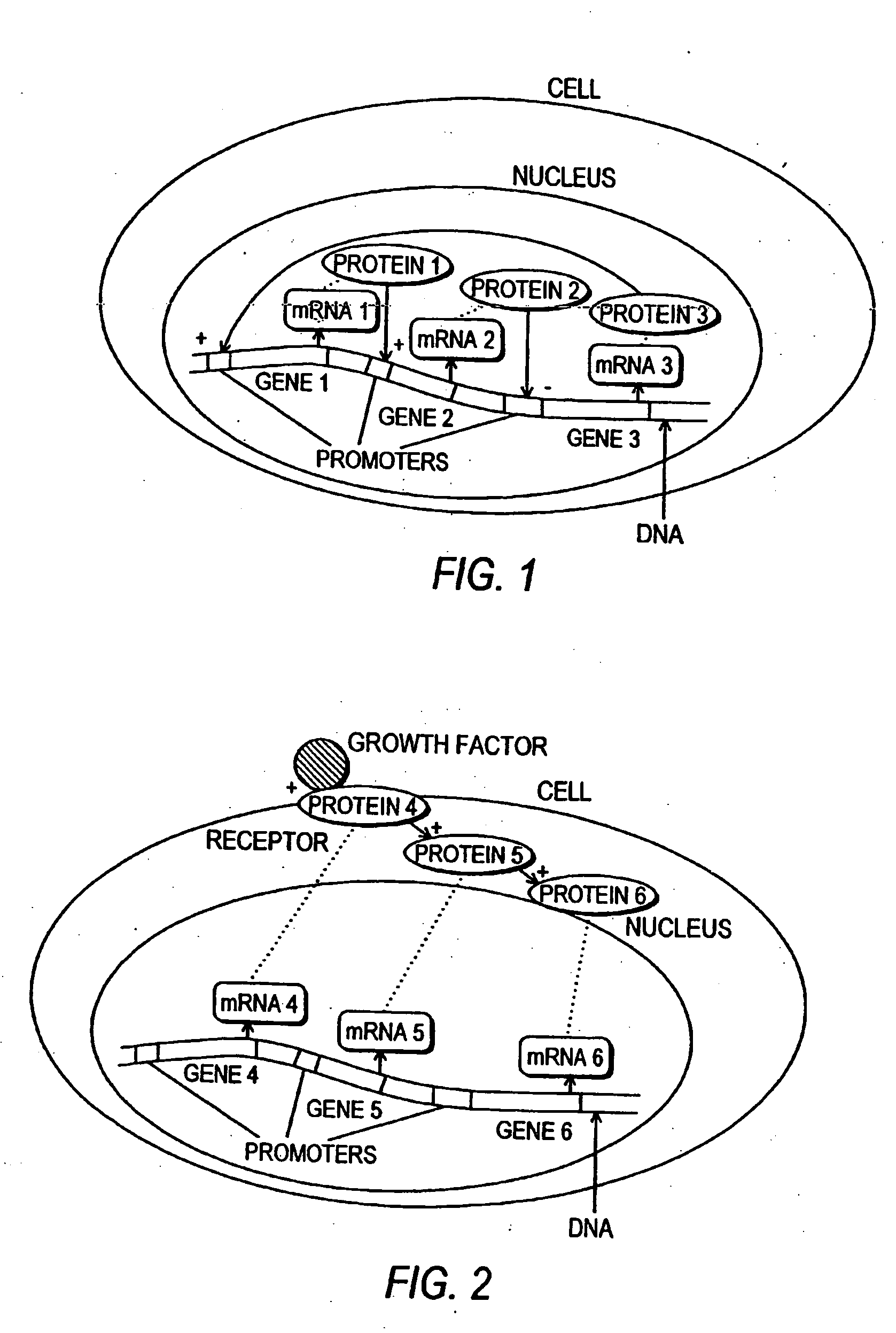
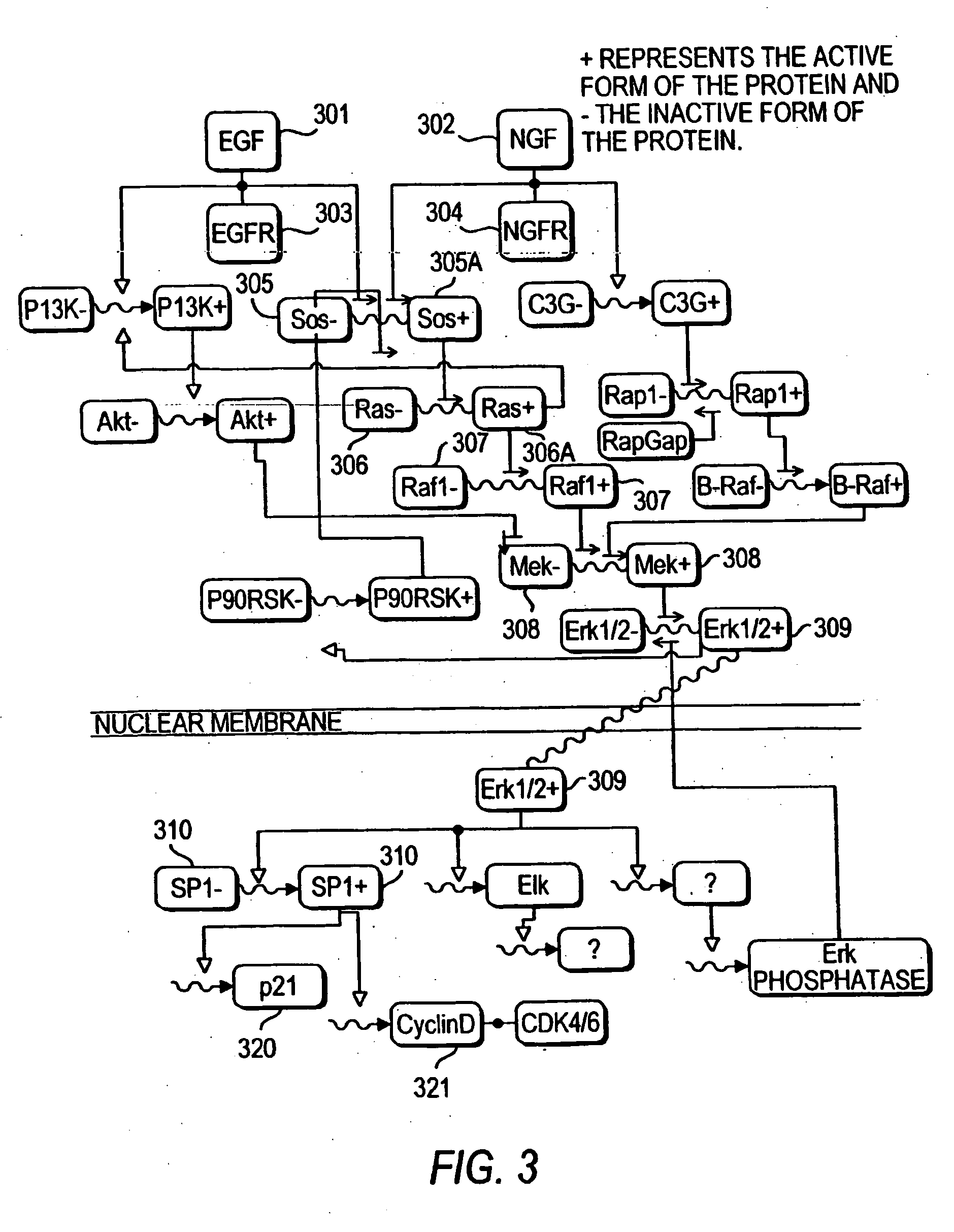
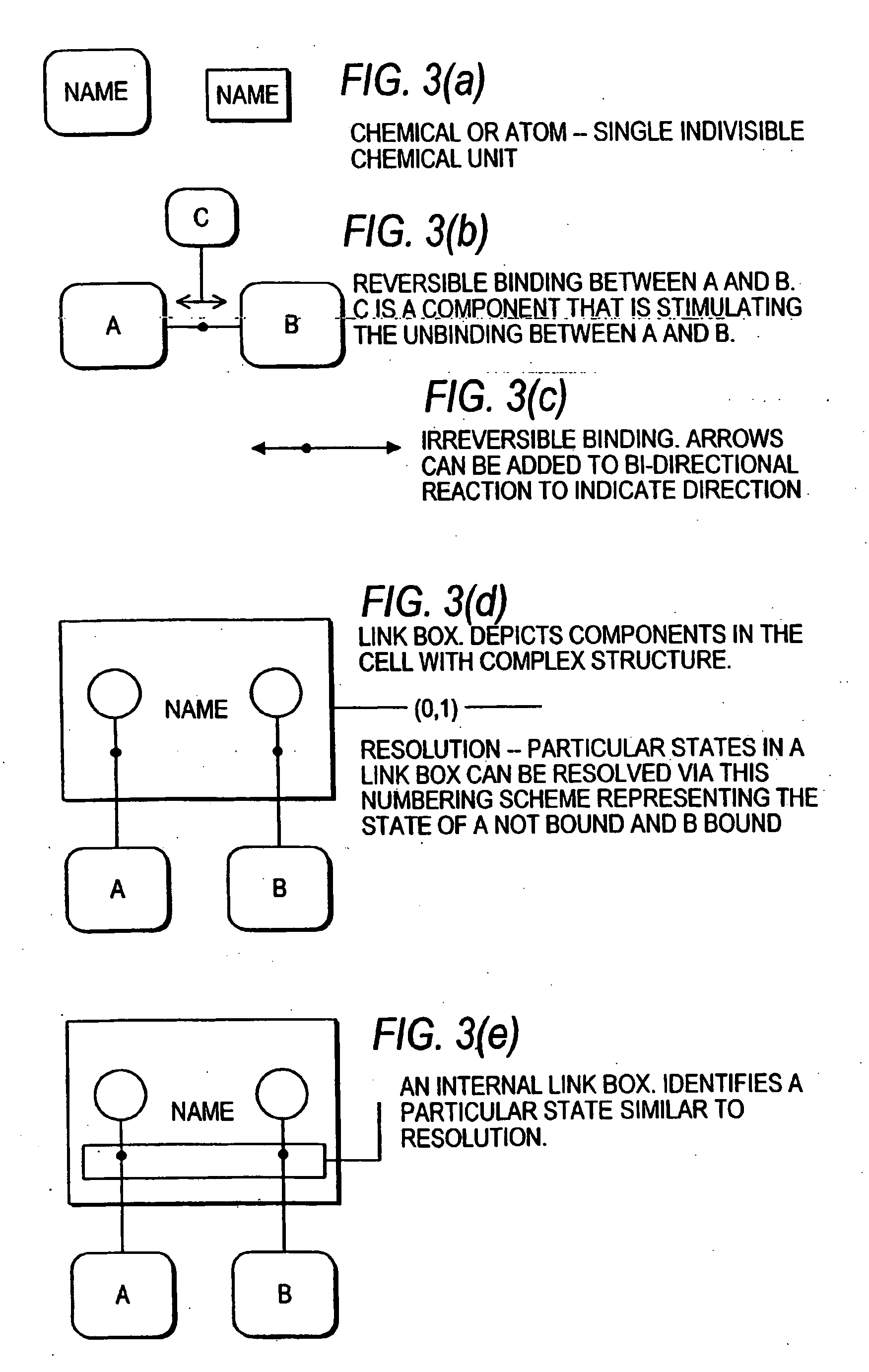
Methods and systems for the identification of components of mammalian biochemical networks as targets for therapeutic agents
a biochemical network and component technology, applied in the field of drug discovery, can solve the problems of limited brute force screening methods, basic empirical methods, and researchers without fundamental information about the mechanism of interaction of compounds, and achieve the effect of avoiding costs and delays
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Description of a Network Comprising Two Genes
[0222]FIG. 5 is a DCL schematic representation of a network comprising two genes, GA and GB. GA and GB are transcribed independently from two separate promoters, PA and PB, to produce mRNA A and mRNA B, respectively, which are then translated to produce proteins A and B, respectively. Transcription and translation are approximated as a single process. Protein A inhibits the production of B. Proteins A and B together activate the production of A. This is only physically plausible if the operator DNA sequences in promoters PA and PB are similar. PAtotal and PBtotal represent the total number of promoter copies for genes A and B and is equal to one for a single copy of the gene circuit. Promoter A7PA7 controls the production of protein A from gene A7GA. Promoter B7P controls production of protein B from gene B. Protein A represses production of protein B (indicated by −) while protein A and protein B together activate the production of prot...
example ii
Description of the Wnt β Catenin Pathway
[0249]FIG. 10 contains a graphical representation of the Wnt β-catenin pathway indicating the role of Axin, APC, and GSK3 in phosphorylating β-catenin and leading to its degradation. FIG. 10 was created as well using Diagrammatic Cell Language, which was discussed above in connection with FIG. 3.
[0250] In FIG. 10, there are two broad horizontal lines, CM and NM. The upper broad line CM represents schematically the cell membrane; that is, the outer membrane of the cell, and the lower broad line NM represents the nuclear membrane of the cell. Elements below the line NM are in the nucleus, and elements above the line CM are outside of the cell.
[0251] Referring again to FIG. 10, Wnt signaling is induced by secreted Wnt proteins that bind to a class of seven-pass transmembrane receptors encoded by the frizzled genes. Activation of the frizzled receptor leads to the phosphorylation of disheveled (Dsh) through an unknown mechanism. Activated dishe...
example iii
[0270] One or more components of a cell can be identified as putative targets for interaction with one or more agents within the simulation. This is achieved by perturbing the simulated network by deleting one or more components thereof, changing the concentration of one or more components thereof or modifying one or more of the mathematical equations representing interrelationships between two or more of said components. Alternatively, the concentrations of one or more of the several proteins and genes in the biochemical network are selectively perturbed to identify which ones of said proteins or genes cause a change in the time course of the concentration of a gene or protein implicated in a disease state of the cell.
[0271] Deleting One or More Components in the Network
[0272] The APC protein is deleted by removing the protein from the set of equations in Example II and removing all of the chemical species formed and reactions that take place as a result of interactions with APC....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com