Oral compositions having increased bioavailability and methods of using the same
a technology of oral compositions and retinoids, which is applied in the directions of drug compositions, dispersed delivery, biocides, etc., can solve the problem of limited bioavailability of oral fenretinide compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
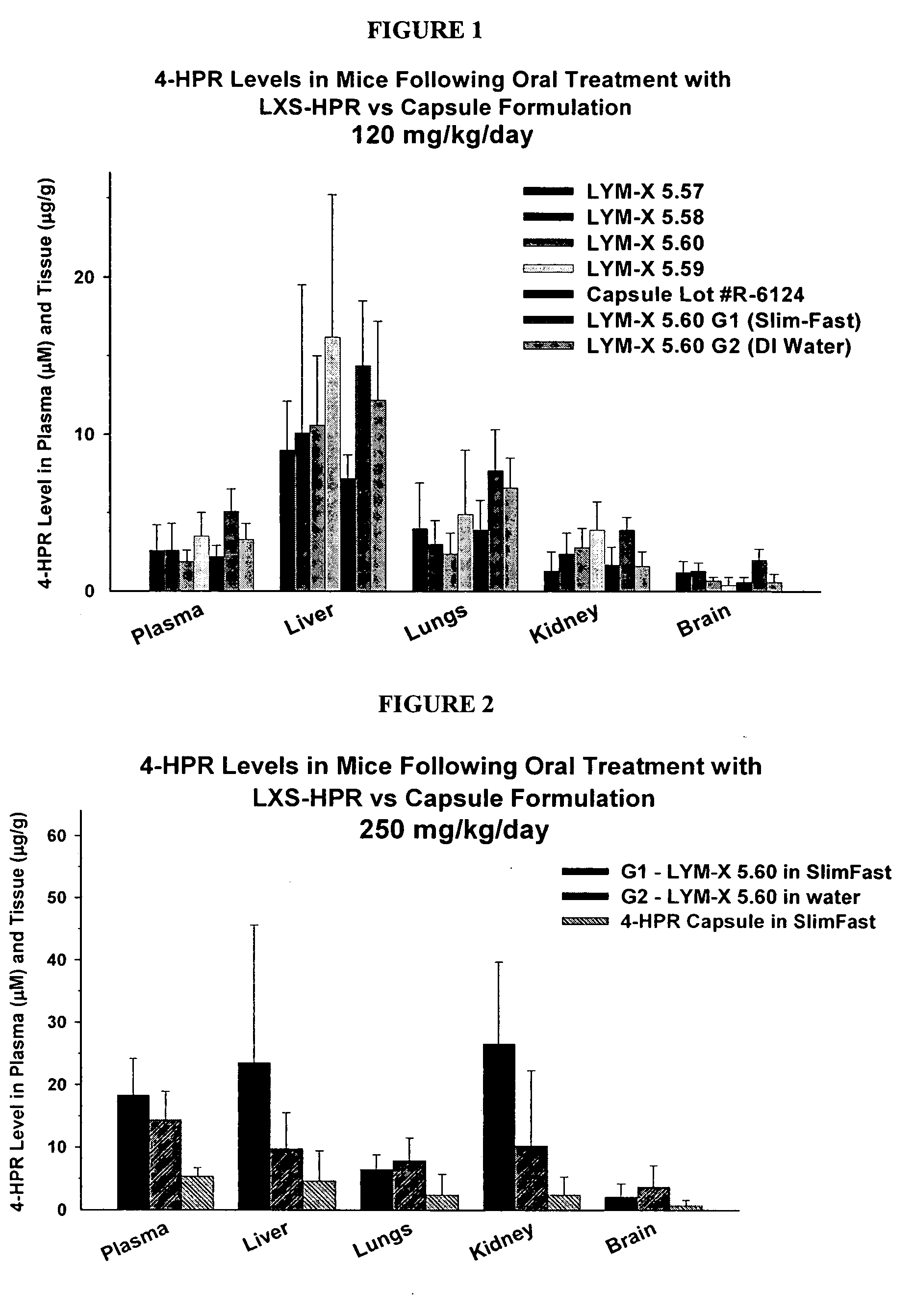
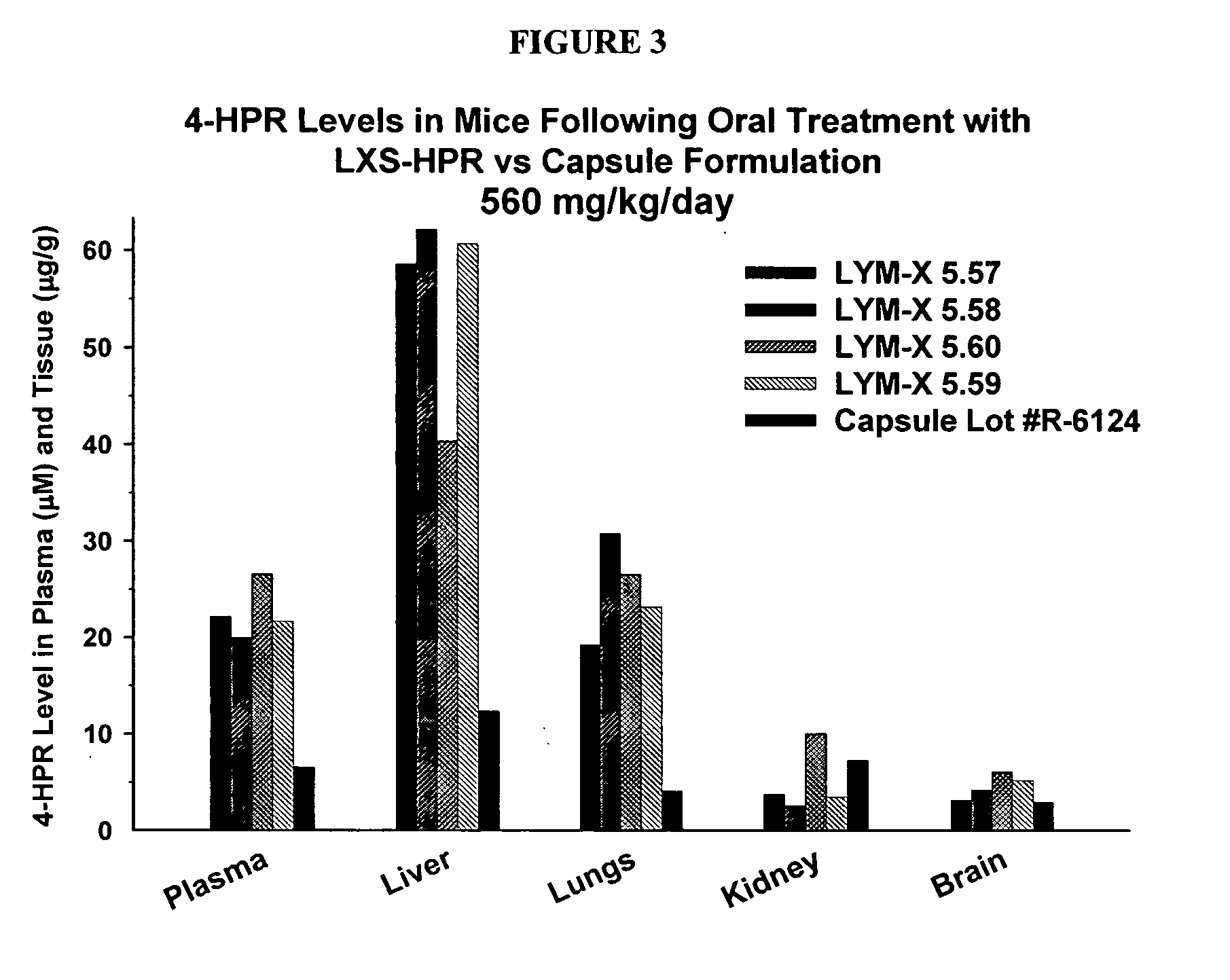
Oral Compositions of 4-HPR
[0063] The actual dose of 4-HPR to be delivered to patients will ultimately define the resulting mixture volume, once it is determined. These arbitrary values present 4-HPR doses of 0.5 to 2.5 grams per mixture volume.
[0064] A concentrated LXS-HPR mixture would be >1000 mg HPR per 10 cc. As awkward as this may be for typical dosage calculations, we usually describe [drug]-LXS ratios in terms of moles. This is because our prior work suggests that 1 mole of drug successfully interacts within the inclusion space of 1 mole of LXS. So, if we use a mole ratio of 0.8 (0.8 moles of drug per mole of LXS), the maximum concentrated dosage would be 397*0.8 / 2429, or 131 mg HPR per gram of LXS.
[0065] In reading Table 1, then, with a mole ratio of 0.8, 2.5 g of 4-HPR would complex with 19.09 g LXS with a resulting total volume of 17.18 ml, or approximately 1400 mg per 10 cc. The calculations for lower mole ratios are included.
[0066] Preferably, the LXS composition (in...
example 2
Improved Bioavailability of Oral 4-HPR Using LYM-X-SORB™ Lipid Matrix
[0067] LYM-X-SORB™ (Lymphatic Xenobiotic Absorbability) lipid matrix (also referred to as LXS™ herein) is a patented and proprietary technology of LYM-DRUG PRODUCTS, LLC and BioMolecular Products, Inc., Byfield, Mass., useful for increasing the oral bioavailability of water insoluble compounds. LXS™ is a non-liposomal, lipid-based, drug delivery system composed of FDA-accepted GRAS (Generally Regarded As Safe) lipids (lysophosphatidylcholine (LPC), monoglycerides (MG), and fatty acids (FA)). LXS™ acts as a “lipid-fingered glove” which wraps around the drug of interest in a 1:1 molar ratio. In the presence of sodium bicarbonate and bile salts, the LXS / drug matrix forms <10 nm size particles that are readily absorbed. Neither the LXS™ monomer, nor the LXS / drug matrix, damage intestinal villae. LXS™ has been demonstrated safe in a one-year double blind oral feeding trial in Cystic Fibrosis (CF) children (Lepage, et a...
example 3
Powder Formulation Process
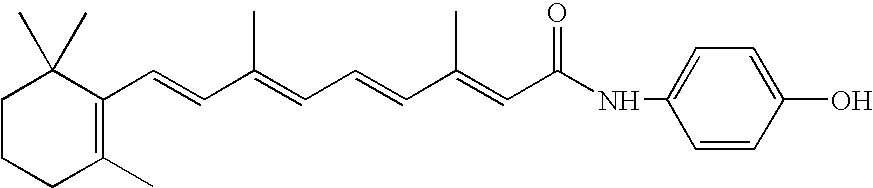
[0074] A 1:0.8 Mole ratio combination of LYM-X-SORB™ drug matrix compostition was combined with fenretinide in accordance with known techniques and as described in Example 1 above in a 1:0.8 Mole ratio to form an LXS / 4-HPR complex. The lipid matrix is not acidified or significantly hydrated to form a protonated aqueous lipid matrix of predominantly hexagonal or inverse hexagonal phase. Rather, the lipid matrix is basic, minimally-hydrated, and of predominantly lamellar phase. The LXS / 4-HPR is blended to anoil and stored at −20° C. The oil is ˜88.8% (wt) LXS and 11.2% (wt) 4-HPR (1:0.8 mole ratio).
[0075] A food processor is used for the blending to a powder but this may be replaced by a much larger reactor vessel with internal chopper blades or other mixing device to process multiple kilos simultaneously. Either way, the flour and sugar are loaded into the blending vessel and mixed for ˜1 minute or more at room temperature. The LXS / 4-HPR is then loaded at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com