Apparatuses and methods for atomic layer deposition of hafnium-containing high-k dielectric materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
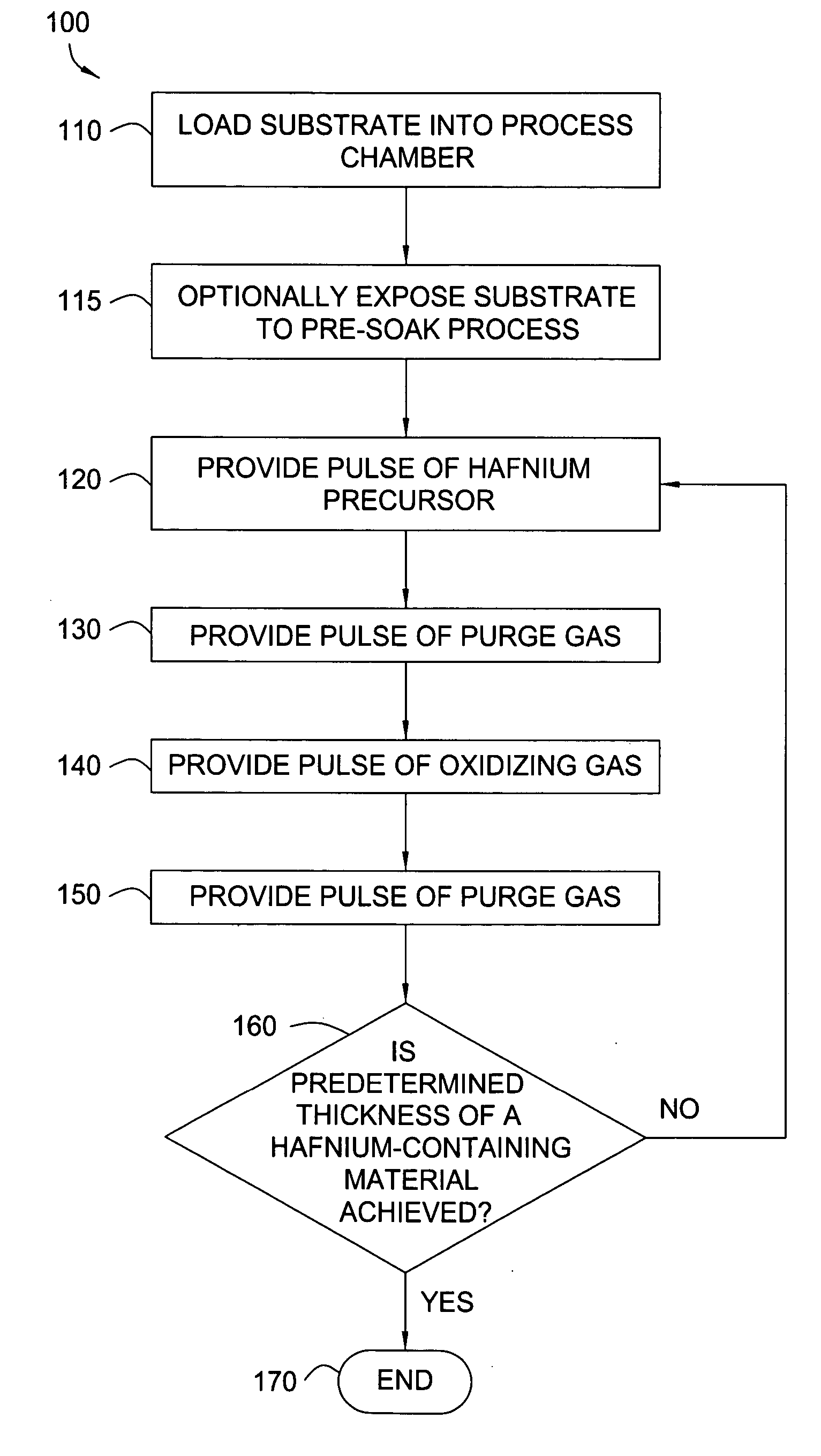
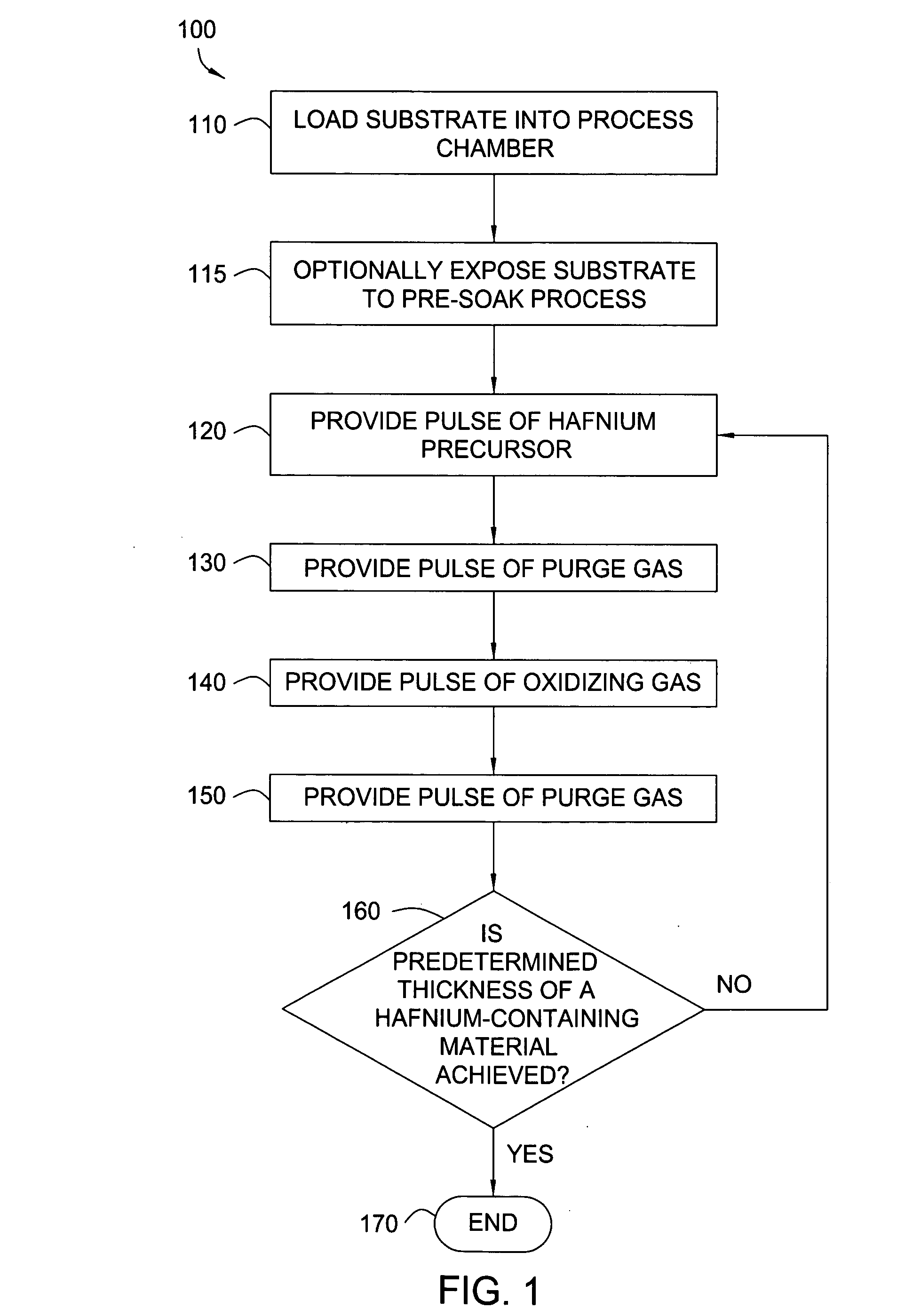
[0129] A hafnium oxide film is formed during an ALD process by sequentially pulsing a hafnium precursor with an oxidizing gas produced by a WVG system. A substrate surface is exposed to a pretreatment process to form hydroxyl groups thereon. The hafnium precursor, HfCl4, is heated within a precursor ampoule at a temperature in a range from about 150° C. to about 200° C. A nitrogen carrier gas is directed into the precursor ampoule containing the hafnium precursor with a flow rate of about 400 sccm. The hafnium precursor saturates the carrier gas and is provided into the chamber for about 3 seconds. A purge gas of nitrogen is provided into the chamber for about 2.5 seconds to remove any unbound hafnium precursor. Hydrogen gas and oxygen gas with the flow rate of about 100 sccm and about 120 sccm respectively, are supplied to the WVG system. The oxidizing gas coming from the WVG system contains water with a flow rate of about 100 sccm and oxygen with a flow rate of about 70 sccm. The ...
example 2
[0130] A hafnium oxide film is formed during an ALD process by sequentially pulsing a hafnium precursor with an oxidizing gas. A substrate surface is exposed to a pretreatment process to form hydroxyl groups thereon. The hafnium precursor, HfCl4, is heated within a precursor ampoule at a temperature in a range from about 150° C. to about 200° C. A nitrogen carrier gas is directed into the precursor ampoule containing the hafnium precursor with a flow rate of about 400 sccm. The hafnium precursor saturates the carrier gas and is provided into the chamber for about 0.5 seconds. A purge gas of nitrogen is provided into the chamber for about 0.5 seconds to remove any unbound hafnium precursor. Hydrogen gas and oxygen gas with the flow rate of about 50 sccm and about 60 sccm respectively, are supplied to the WVG system. The oxidizing gas coming from the WVG system contains water with a flow rate of about 50 sccm and oxygen with a flow rate of about 35 sccm. The oxidizing gas is provided ...
example 3
[0131] A hafnium silicate film is formed during with an ALD process by sequentially pulsing a hafnium precursor with an oxidizing gas followed by pulsing a silicon precursor with the oxidizing gas. A substrate surface is exposed to a pretreatment process to form hydroxyl groups thereon. The hafnium precursor, TDEAH, and silicon precursor, TDMAS, are heated within separate precursor ampoules at room temperature (about 23° C.). These precursors are vaporized individually in vaporizers at about 110° C. to about 130° C. and individually mixed with an inert carrier gas. The hafnium precursor saturates the carrier gas and is provided into the chamber for about 1 second. A purge gas of nitrogen is provided into the chamber for about 1 second to remove any unbound hafnium precursor. Hydrogen gas and oxygen gas with the flow rate of about 100 sccm and about 120 sccm respectively, are supplied to the WVG system. The oxidizing gas coming from the WVG system contains water with a flow rate of a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Dielectric polarization enthalpy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com