Cobalt-nickel-chromium biocompatible alloy for implantable medical devices
a biocompatible, medical device technology, applied in the field of cobaltnickelchromium biocompatible alloys for implantable medical devices, can solve the problems of inability to use some vessels, affecting the patient's life, and affecting the patient's health, and achieves the effect of simple and inexpensive manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
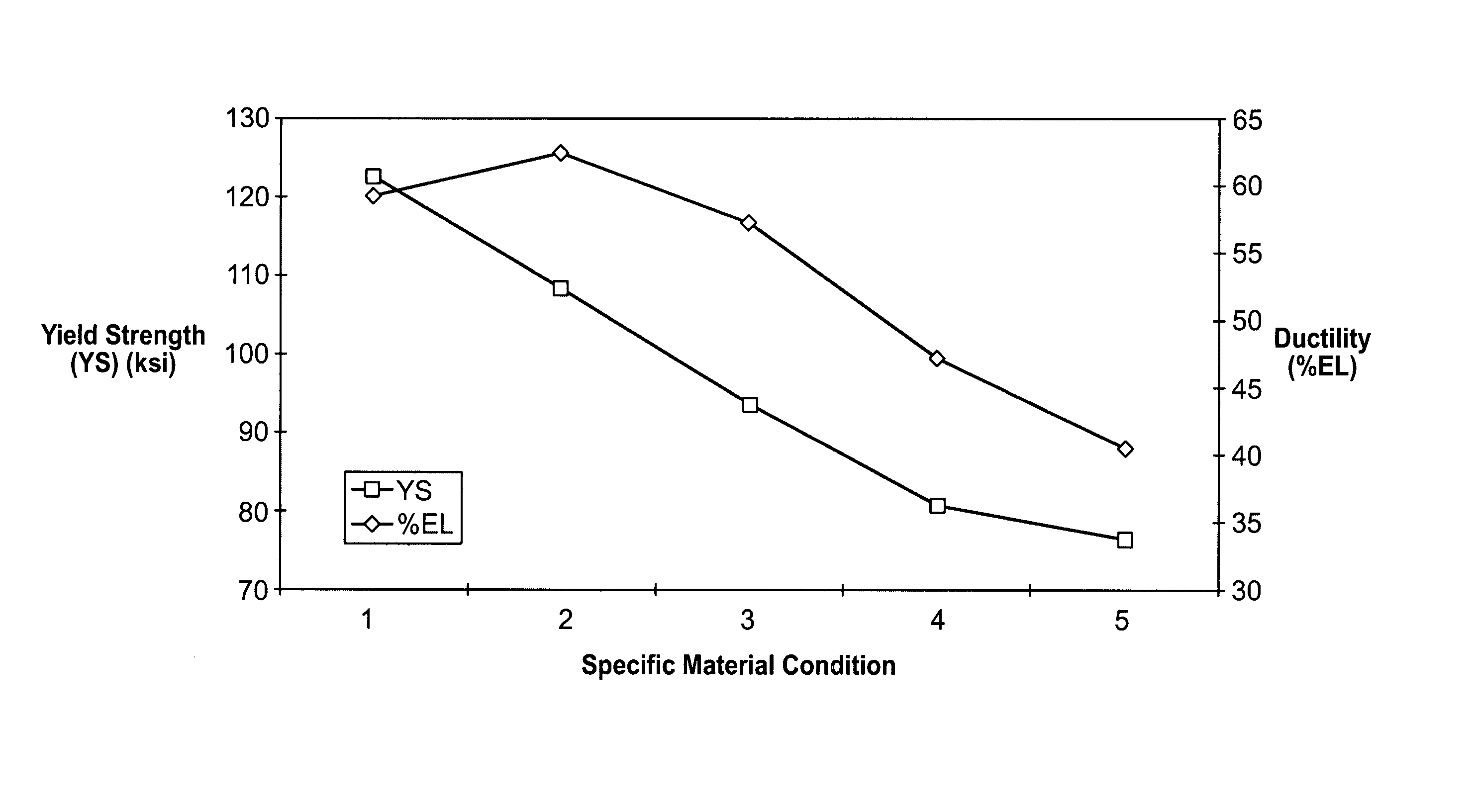
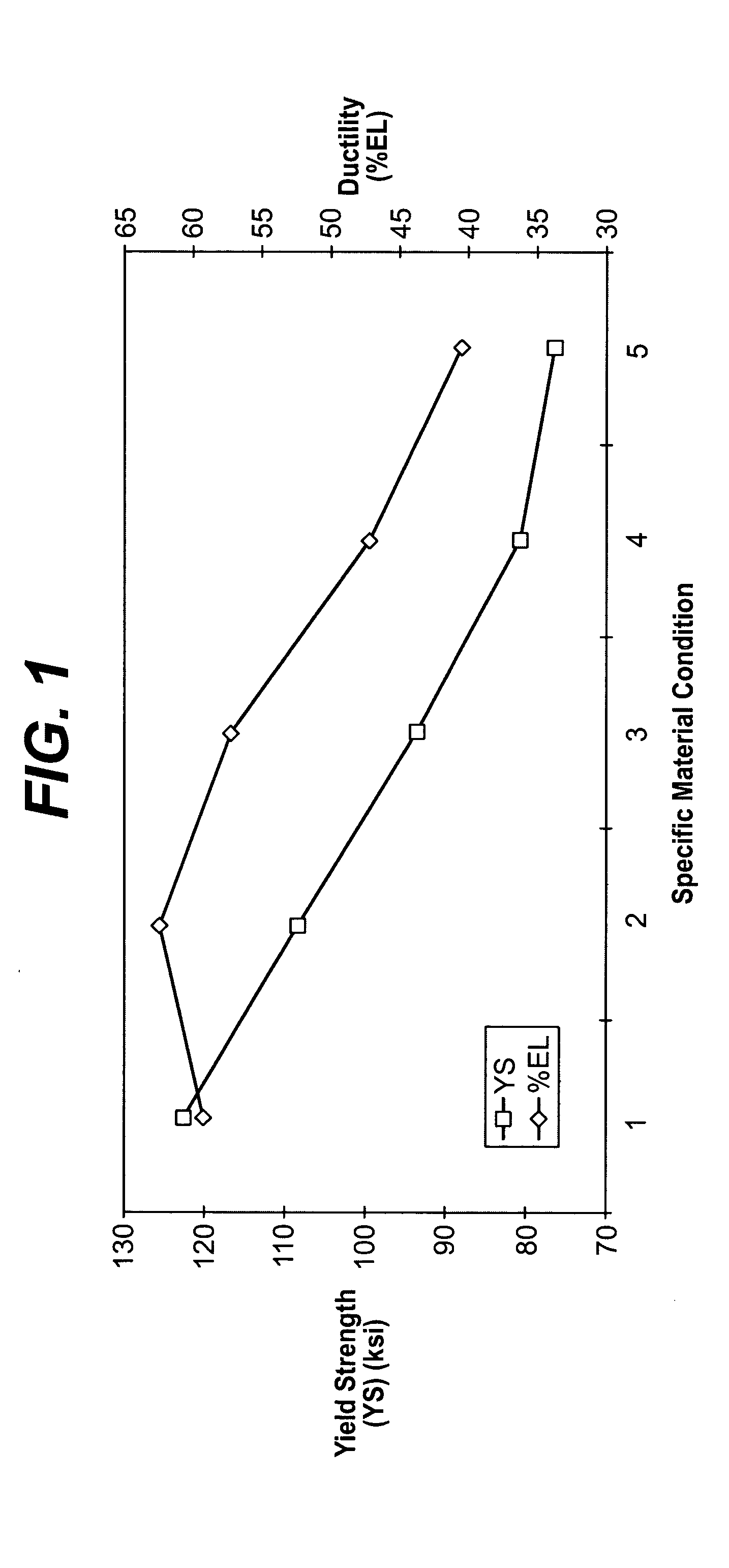
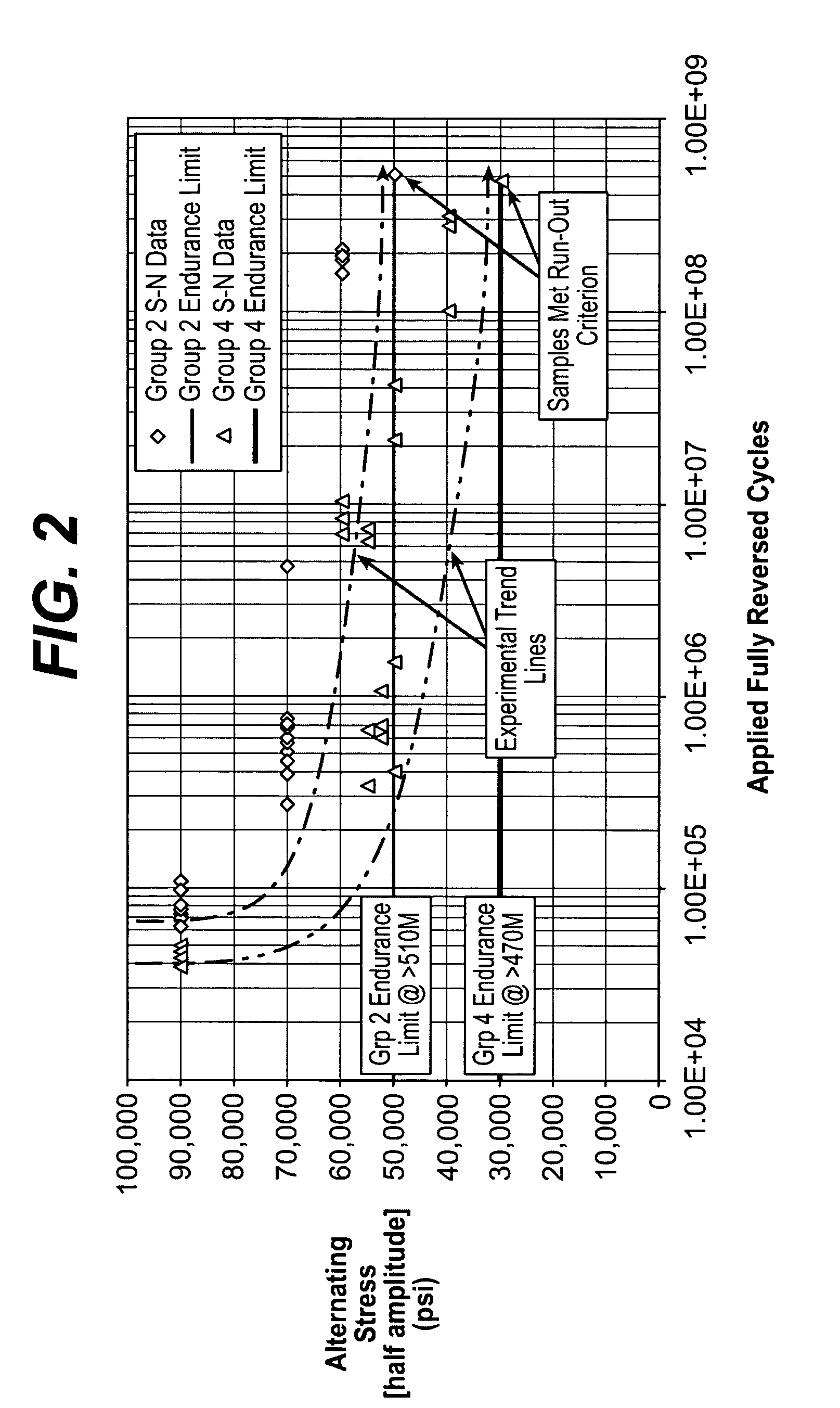
[0025] Biocompatible, solid-solution alloys may be utilized in the manufacture of any number of implantable medical devices. The biocompatible alloy for implantable medical devices in accordance with the present invention offers a number of advantages over currently utilized medical grade alloys. In particular, the biocompatible alloy of the present invention is magnetic resonance imaging compatible. Magnetic resonance imaging is a valuable diagnostic tool and thus any implantable medical device should preferably be magnetic resonance imaging compatible so that it and surrounding tissue may be accurately imaged. One such medical device where this is particularly relevant is stents.
[0026] Coronary stenting is currently the most widely utilized percutaneous coronary intervention. The primary benefit of stenting when compared with balloon-angioplasty alone is a reduction of the restenosis-rate. Nevertheless, in-stent restenosis remains a relatively common clinical scenario. If clinica...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com